��Ŀ����
�ɶ���һ�ּ���ʳƷ�������з����һ����C��H��O����Ԫ����ɵ��л���A��Ϊȷ����ṹ�ֽ������¸�ʵ�飺
��6.0g A��һ����������ȫ�ֽ⣬����3.36L����״���£�һ����̼��1.8gˮ��
���к�0.24g����A����0.2mol/L��NaOH��Һ20.00mL��
��0.01mol����A��ȫת��Ϊ������Ҫ�Ҵ�0.92g��0.01mol A���������Ʒ�Ӧ�ų�0.336L����״���£�������
��ͨ������ȷ������1��A�Ļ�ѧʽ����2��A�Ľṹ��ʽ��
��6.0g A��һ����������ȫ�ֽ⣬����3.36L����״���£�һ����̼��1.8gˮ��
���к�0.24g����A����0.2mol/L��NaOH��Һ20.00mL��
��0.01mol����A��ȫת��Ϊ������Ҫ�Ҵ�0.92g��0.01mol A���������Ʒ�Ӧ�ų�0.336L����״���£�������
��ͨ������ȷ������1��A�Ļ�ѧʽ����2��A�Ľṹ��ʽ��
��������1��0.01mol����A��ȫת��Ϊ������Ҫ�Ҵ�0.02mol����A�����к���2��-COOH����ȫ�к�0.24g����A����Ҫ����0.004molNaOH����0.24gA�����ʵ���Ϊ0.002mol���ݴ˼����л���A��Ħ��������
����6.0gA�����ʵ�������һ����������ȫ�ֽ⣬����3.36L����״���£�һ����̼��1.8gˮ������CO��H2O�����ʵ���������Ԫ���غ㣬�����л���A�Ļ�ѧʽ��
��2��0.01mol����A��ȫת��Ϊ������Ҫ�Ҵ�0.02mol����A�����к���2��-COOH��0.01molA���������Ʒ�Ӧ�ų�0.336L����״���£����������������ʵ���Ϊ0.015mol��0.01molA�ṩ0.03molHԭ�ӣ������л����л�����1��-OH������л���ķ���ʽ��д���ṹ��ʽ��
����6.0gA�����ʵ�������һ����������ȫ�ֽ⣬����3.36L����״���£�һ����̼��1.8gˮ������CO��H2O�����ʵ���������Ԫ���غ㣬�����л���A�Ļ�ѧʽ��
��2��0.01mol����A��ȫת��Ϊ������Ҫ�Ҵ�0.02mol����A�����к���2��-COOH��0.01molA���������Ʒ�Ӧ�ų�0.336L����״���£����������������ʵ���Ϊ0.015mol��0.01molA�ṩ0.03molHԭ�ӣ������л����л�����1��-OH������л���ķ���ʽ��д���ṹ��ʽ��
����⣺��1��0.01mol����A��ȫת��Ϊ������Ҫ�Ҵ�Ϊ
=0.02mol����A�����к���2��-COOH����ȫ�к�0.24g����A����Ҫ����NaOHΪ0.02L��0.2mol/L=0.004mol����0.24gA�����ʵ���Ϊ0.002mol���л���A��Ħ������Ϊ
=120g/mol��
6.0gA�����ʵ���Ϊ
=0.05mol����һ����������ȫ�ֽ⣬����3.36L����״���£�һ����̼�����ʵ���Ϊ
=0.15mol������1.8gˮ��ˮ�����ʵ���Ϊ
=0.1mol��
����Ԫ���غ��֪0.05molA����0.15molC��0.2molH��Oԭ��Ϊ0.15mol+0.1mol=0.25mol��
��1molA����3molC��4molH��5molOԭ�ӣ��л���A�ķ���ʽΪC3H4O5��
���л���A��Ħ������Ϊ120g/mol����ѧʽΪC3H4O5��
��2��0.01mol����A��ȫת��Ϊ������Ҫ�Ҵ�0.02mol����A�����к���2��-COOH��0.01molA���������Ʒ�Ӧ�ų�0.336L����״���£����������������ʵ���Ϊ0.015mol��0.01molA�ṩ0.03molHԭ�ӣ������л����л�����1��-OH���л���ķ���ʽΪC3H4O5�����л���A�Ľṹ��ʽΪ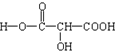 ��
��
���л���A�Ľṹ��ʽΪ ��
��
| 0.92g |
| 46g/mol |
| 0.24g |
| 0.002mol |
6.0gA�����ʵ���Ϊ
| 6.0g |
| 120g/mol |
| 3.36L |
| 22.4L/mol |
| 1.8g |
| 18g/mol |
����Ԫ���غ��֪0.05molA����0.15molC��0.2molH��Oԭ��Ϊ0.15mol+0.1mol=0.25mol��
��1molA����3molC��4molH��5molOԭ�ӣ��л���A�ķ���ʽΪC3H4O5��
���л���A��Ħ������Ϊ120g/mol����ѧʽΪC3H4O5��
��2��0.01mol����A��ȫת��Ϊ������Ҫ�Ҵ�0.02mol����A�����к���2��-COOH��0.01molA���������Ʒ�Ӧ�ų�0.336L����״���£����������������ʵ���Ϊ0.015mol��0.01molA�ṩ0.03molHԭ�ӣ������л����л�����1��-OH���л���ķ���ʽΪC3H4O5�����л���A�Ľṹ��ʽΪ
 ��
�����л���A�Ľṹ��ʽΪ
 ��
�������������л�����ƶϡ��л�������ʵȣ��Ѷ��еȣ����������ƶ��л���Ĺ���������Ŀ�ǽ���Ĺؼ���

��ϰ��ϵ�д�
�����Ŀ