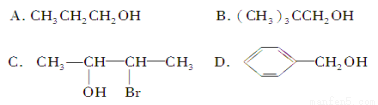��Ŀ����
ij�о���ѧϰС��Ϊ��֤��ij��HmA��ǿ�ỹ�����ᣬ���������ʵ�鷽������ش��й����⣺
��1����m=lʱ���ⶨ0.1 mol/L HA��pHֵ�������0��1mol/L HA��pH=2��˵��HAΪ__________��(����ǿ�����������������Һ�м���ϡNaOH��Һ��ʹ��ǡ����ȫ��Ӧ��������ҺpH_________7������>����<����=��)�������ӷ���ʽ��ʾ��ԭ��__________��
��2��ȡ����NamA������������ˮ����������Һ�еμ�2��ʯ����Һ������Һ����ɫ�����ɫ��֤��HmA��_______�ᣨ����ǿ����������������ijŨ�ȵ�NamA��ҺpH=9�������Һ��ˮ�����c��OH����=_________mol/L��
��3�������£���pH=2����HmA��pH=12�ļ�B(OH)n��������,��û��Һ��pH=10,�����ɵ�������ֻ��һ��������ˮ�⡣�������ˮ������ӷ���ʽΪ_________________________����m��n��Ϊ1�����û��Һ�и����ӵ����ʵ���Ũ���ɴ�С��˳����____________��
��4��������ͬŨ�ȵ�HA1��HA2������Һ������ˮ������ָʾ������ʯ���̪����pH��ֽ�Լ����õ�ʵ������������λͬѧ�������飺һλͬѧȡ����HA1��Һ���μ�2��ʯ����Һ����Һ��졣�ȣ��۲췢����Һ��ɫ����ƶ�HA1Ϊ________ �ᣨ����ǿ����������������һλͬѧȡ����HA2��Һ���ⶨ��pH��Ȼ����Һϡ��100�����ٲ���pH���Ա����βⶨ��pH���2���ݴ��ƶ�HA2Ϊ__________�ᣨ����ǿ��������������
��1���� �� A��+H2O HA+OH����2���� 1��10��5
HA+OH����2���� 1��10��5
��3��Bn++nH2O B(OH)n+nH+ c��B+����c��A-����c��OH-����c��H+����4���� ǿ
B(OH)n+nH+ c��B+����c��A-����c��OH-����c��H+����4���� ǿ
��������
���������֤��HmAΪ���ᣬ�ɴ����½Ƕ��жϣ���������ˮ��Һ�еĵ���̶ȣ������ֵ��룬��Ϊ��������Ƿ���ڵ���ƽ�⣬�����ڵ���ƽ�⣬��Ϊ�������NamA��Һ������ԣ����Լ��ԣ���Ϊ�����1����m=lʱ���ⶨ0.1 mol/L HA��pHֵ�������0��1mol/L HA��pH=2����HA���ֵ��룬��˵��HA����������Һ�м���ϡNaOH��Һ��ʹ��ǡ����ȫ��Ӧ��������ҺΪNaA��Һ��Ϊǿ����������Һ��ˮ���Լ��ԣ�pH��7�������ӷ���ʽ��ʾ��ԭ��A��+H2O HA+OH������2��ȡ����NamA������������ˮ����������Һ�еμ�2��ʯ����Һ������Һ����ɫ�����ɫ��˵��NamA��Һ�Լ�����NamAΪǿ��������ˮ���Լ��ԣ�֤��HmA����������ijŨ�ȵ�NamA��ҺpH=9������Һ��c��H+��=1��10��9mol/L��c��OH-��=1��10��5mol/L������Һ��H+��OH��������ˮ�ĵ��롣�����Һ��ˮ�����c��OH����=1��10��5mol/L����3�������£���pH=2����HmA��pH=12�ļ�B(OH)n��������,��û��Һ��pH=10���Լ��ԣ�����߷�Ӧ�������˵��HmAΪǿ�ᣬB(OH)nΪ������ɵ�������ֻ��Bn+��ˮ����ˮ������ӷ���ʽΪBn++nH2O
HA+OH������2��ȡ����NamA������������ˮ����������Һ�еμ�2��ʯ����Һ������Һ����ɫ�����ɫ��˵��NamA��Һ�Լ�����NamAΪǿ��������ˮ���Լ��ԣ�֤��HmA����������ijŨ�ȵ�NamA��ҺpH=9������Һ��c��H+��=1��10��9mol/L��c��OH-��=1��10��5mol/L������Һ��H+��OH��������ˮ�ĵ��롣�����Һ��ˮ�����c��OH����=1��10��5mol/L����3�������£���pH=2����HmA��pH=12�ļ�B(OH)n��������,��û��Һ��pH=10���Լ��ԣ�����߷�Ӧ�������˵��HmAΪǿ�ᣬB(OH)nΪ������ɵ�������ֻ��Bn+��ˮ����ˮ������ӷ���ʽΪBn++nH2O B(OH)n+nH+����m��n��Ϊ1�����û��Һ�Լ��ԣ���c��OH-����c��H+�������ݵ���غ�֪��c��B+����c��A-���������ӵ����ʵ���Ũ���ɴ�С��˳����c��B+����c��A-����c��OH-����c��H+������4��ȡ����HA1��Һ���μ�2��ʯ����Һ����Һ��졣�ȣ��۲췢����Һ��ɫ���˵��HA1��Һ�д��ڵ���ƽ�⣬������ʵĵ������ȣ����£��ٽ����룬��ɫ����ƶ�HA1Ϊ������ȡ����HA2��Һ���ⶨ��pH��Ȼ����Һϡ��100�����ٲ���pH���Ա����βⶨ��pH���2���ݴ��ƶ�HA2Ϊǿ�ᡣ
B(OH)n+nH+����m��n��Ϊ1�����û��Һ�Լ��ԣ���c��OH-����c��H+�������ݵ���غ�֪��c��B+����c��A-���������ӵ����ʵ���Ũ���ɴ�С��˳����c��B+����c��A-����c��OH-����c��H+������4��ȡ����HA1��Һ���μ�2��ʯ����Һ����Һ��졣�ȣ��۲췢����Һ��ɫ���˵��HA1��Һ�д��ڵ���ƽ�⣬������ʵĵ������ȣ����£��ٽ����룬��ɫ����ƶ�HA1Ϊ������ȡ����HA2��Һ���ⶨ��pH��Ȼ����Һϡ��100�����ٲ���pH���Ա����βⶨ��pH���2���ݴ��ƶ�HA2Ϊǿ�ᡣ
���㣺����ǿ��������жϵ�ʵ�����ݡ�