��Ŀ����
ij��ɫ��Һ�к���NH4+��K+��Al3+��SO42���������ӣ���������ʵ�飺
��1��ȡ10 mL����Һ���Թ��в��μ�Ba(NO3)2��Һ����ϡ�����ữ����˵õ�0.03 mol��ɫ������
��2��ȡ10 mL����Һ���Թ��У��μ�NaOH��Һ������ɫ���������������ӵ�һ������ʼ��������(��Ҫʱ�ɼ���)����������ȫ�ܽ⡣������NaOH��Һ�ļ��룬����������ı仯��ϵ����ͼ��ʾ�� 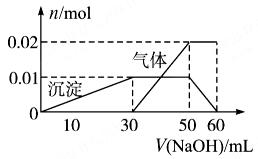
����˵����������ʵ���ǣ� ��
| A������ͼ��ʾ���ݼ���ʵ����ʹ�õ�NaOH��Һ�����ʵ���Ũ��Ϊ1mol/L |
| B��������Ϣ��������Һ����ɫ��Ӧ������ɫ�����ӵ����ʵ���Ũ��Ϊ1mol/L |
| C����ȡһ��������Һ�μ�һ������Ba(OH)2��Һ����ʹAl3+��SO42��ͬʱ��ȫ���� |
| D��NH4+��K+��Al3+��SO42���������ӵ����ʵ���֮��Ϊ��2��1��1��3 |
C
���������������1���а�ɫ����Ϊ���ᱵ������������Ϊ0.03mol������Һ�к����������������������ʵ���Ϊ0.03mol����2����ȡ10mL����Һ���Թ��У��μ�NaOH��Һ������ɫ��������˵����Һ�к���Al3+��Al3++3OH-=Al(OH)3�������ͼ���г����ı仯��ϵ��֪��Һ�к���Al3+Ϊ0.01mol��OH-Ϊ0.03mol�����������ӵ�һ������ʼ�������壬����NH4++OH-=NH3��+H2O���ͼ��֪��Һ�к���NH4+Ϊ0.02mol��OH-Ϊ0.02mol����������ȫ�ܽ�����������������NaOH������Ӧ����Ӧ�����ӷ���ʽΪAl��OH��3+OH-=AlO2-+2H2O�����ͼ���г����ı仯��ϵ����OH-Ϊ0.01mol��ʵ����ʹ�õ�NaOH��Һ�������ʵ���Ϊ��0.03mol+0.02mol+0.01mol=0.06mol�����ͼ���г����ı仯��ϵ֪��ʱ����������Һ�����Ϊ60mL����c��NaOH��=1mol/L��Aѡ���ȷ��Bѡ����ڸ���Һ�к���K+��NH4+��Al3+��SO42-������NH4+Ϊ0.02mol��Al3+Ϊ0.01mol��SO42-Ϊ0.03mol��������Һ�ʵ�������Һ�����������������������������ȣ��������������Ϊ��0.02mol��1+0.01mol��3+n��K+����1=0.05mol+n��K+�������������0.03mol��2=0.06mol������n��K+��=0.01mol������Ũ��Ϊ1mol/L����ȷ��Cѡ�����������֪Al3+Ϊ0.01mol�������Ϊ0.03mol����ʹ��ͬʱ��ȫ����������Ba2+0.03mol��OH-=0.03mol�����Լ���Ba(OH)2����ʽ��ɡ�������Dѡ���ȷ��
���㣺���Ӽ��飬�����㡣

�����£����и���������ָ����Һ��һ���ܴ����������
| A����ʹ��̪������Һ��Na+��Ba2+��NO3����Cl�� |
| B������0.1 mol��L-1 Fe3������Һ�У�K����Mg2����I����SO42�� |
| C�����ܽ�Al(OH)3����Һ��NH4+��K+��SO42����HCO3�� |
| D��c(Al3+)="0.5" mol��L-1����Һ�У�Na+��K+��[Al(OH)4]����SO42�� |
��������ˮ��Һ�ܵ��磬�����������ڷǵ���ʵ���
| A��Na2O | B��Cl2 | C��H2SO4 | D��CO2 |
W��X��Z��ԭ���������������ͬһ������Ԫ�أ�W��X�ǽ���Ԫ�أ�Z�Ƿǽ���Ԫ�أ�W��X������������Ӧ��ˮ����������Ӧ�����κ�ˮ����һ������W������������Ӧ��ˮ������Һ����μ���XZ3��Һ�����ɵij���X(OH)3��������XZ3��Һ�������ı仯��ϵ��ͼ��ʾ���������������ڶ�Ӧ����Һ��һ���ܴ���������ǣ� ��
| A��d���Ӧ����Һ�У�K+��NH4+��CO32����I�� |
| B��c���Ӧ����Һ�У�Ag+��Ca2+��NO3����Na+ |
| C��b���Ӧ����Һ�У�Na+��S2����SO42����Cl�� |
| D��a���Ӧ����Һ�У�Na+��K+��S042����HCO3�� |
���и�����������Һ���ܹ�����ͨ����������Ӧ��������ܴ������ڵ���
| A��Na+��Ba2+��HSO3-��I-�������� |
| B��Ca2+��NO3-��Na+��Cl-���������� |
| C��Fe3+��SO42-��CO32-��NO3- �������� |
| D��Na+��K+��HCO3-��Cl-��������̼�� |
��Һ�п��ܺ���H+��Na+��NH4+��Mg2+��Fe3+��Al3+��SO42-��HCO3-�����ӡ��������Һ�м���һ�����ʵ���Ũ�ȵ�NaOH��Һʱ���������ɳ������ʵ�����NaOH��Һ������仯��ͼ����ͼ��ʾ������˵����ȷ���ǣ� ��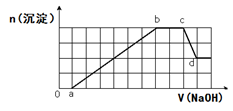
| A��d����Һ�к��е�����ֻ��Na2SO4 |
| B��ab�η��������ӷ�ӦΪ��Al3++3OH-= Al(OH)3��, Mg2++2OH-= Mg(OH)2�� |
| C��ԭ��Һ�к��е���������H+��NH4+��Mg2+��Al3+��Na+ |
| D��ԭ��Һ�к��е�Fe3+��Al3+�����ʵ���֮��Ϊ1:1 |
����״̬�����ʣ����ܵ��������ڵ���ʵ���
| A��KCl��Һ | B��Һ̬HCl | C�����ڵ�NaOH | D��������Һ |
 mol/L��һ���ᷢ����Ӧ����������
mol/L��һ���ᷢ����Ӧ����������