��Ŀ����
I��ʵ������Na2CO3?10H2O��������50g ��������Ϊ21.2%��Na2CO3��Һ���ش��������⣺
��1��Ӧ��������ƽ��ȡNa2CO3?10H2O����
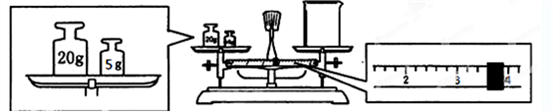
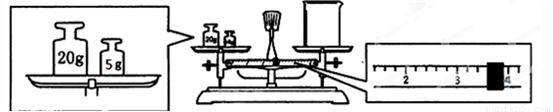
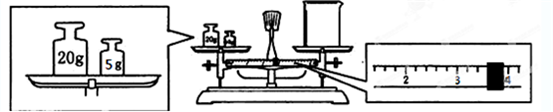
��2����������ƽ��С�ձ��Ƴ�̼���ƾ������������ƽƽ����״̬��ͼ����ͼ�п��Կ�������ͬѧ�ڲ���ʱ����һ��������
II��ʵ����Ҫ����2.5mol/L ��ϡ������Һ90mL���ش��������⣺
��1������Ͳ��ȡ��������Ϊ98%���ܶ�Ϊ1.84g/cm3��Ũ����
��2������ʱ������ʹ�õ���������Ͳ���ձ��⣬��ȱ�ٵ�������
��3��������Һ�Ĺ����У�������������ȷ�����в�����ʹ������ҺŨ��ƫ�ߵ���
A������Ͳ��ȡŨ����ʱ����
B��ϡ������ʱ��δ��ȴ�����¼�ת�Ƶ�����ƿ��
C����ȡŨH2SO4�����Ͳ����ϴ�ӣ�����ϴ��Һת�Ƶ�����ƿ�У�
D������ҡ�Ⱥ���Һ����ڿ̶��ߣ����ý�ͷ�ιܼ�����ˮ����Һ����ʹ���̶�������
E������ƿ�����
��1��Ӧ��������ƽ��ȡNa2CO3?10H2O����
28.6
28.6
g����2����������ƽ��С�ձ��Ƴ�̼���ƾ������������ƽƽ����״̬��ͼ����ͼ�п��Կ�������ͬѧ�ڲ���ʱ����һ��������
�������Ʒλ�õߵ�
�������Ʒλ�õߵ�
��ʵ�ʳ�����̼���ƾ�������Ϊ21.4
21.4
g��
II��ʵ����Ҫ����2.5mol/L ��ϡ������Һ90mL���ش��������⣺
��1������Ͳ��ȡ��������Ϊ98%���ܶ�Ϊ1.84g/cm3��Ũ����
13.6
13.6
mL����2������ʱ������ʹ�õ���������Ͳ���ձ��⣬��ȱ�ٵ�������
����������ͷ�ιܡ�100mL����ƿ
����������ͷ�ιܡ�100mL����ƿ
����3��������Һ�Ĺ����У�������������ȷ�����в�����ʹ������ҺŨ��ƫ�ߵ���
B��C
B��C
��A������Ͳ��ȡŨ����ʱ����
B��ϡ������ʱ��δ��ȴ�����¼�ת�Ƶ�����ƿ��
C����ȡŨH2SO4�����Ͳ����ϴ�ӣ�����ϴ��Һת�Ƶ�����ƿ�У�
D������ҡ�Ⱥ���Һ����ڿ̶��ߣ����ý�ͷ�ιܼ�����ˮ����Һ����ʹ���̶�������
E������ƿ�����
������I����1������Na2CO3�����ʵ�����Na2CO3?10H2O�����ʵ�����ȼ��㣻
��2����ƽƽ��ԭ��Ϊ����������=����������+������ֵ���ݴ˼��㣮
II����1��ʵ������90ml����ƿ���밴100ml����ƿ���㣻
��2������������Һ��ʵ���������ѡ������������
��3��A�����Ӷ������ƫС��
B��Һ��������������
C����Ͳ����ϴ�ӣ�
D������ҡ�Ⱥ������ټ�����ˮ��
E������ƿ�������Ӱ�죻
��2����ƽƽ��ԭ��Ϊ����������=����������+������ֵ���ݴ˼��㣮
II����1��ʵ������90ml����ƿ���밴100ml����ƿ���㣻
��2������������Һ��ʵ���������ѡ������������
��3��A�����Ӷ������ƫС��
B��Һ��������������
C����Ͳ����ϴ�ӣ�
D������ҡ�Ⱥ������ټ�����ˮ��
E������ƿ�������Ӱ�죻
����⣺I����1��ʵ��������50g ��������Ϊ21.2%��Na2CO3��Һ����ҪNa2CO3������Ϊ��50��21.2%=10.6�ˣ�Na2CO3�����ʵ���Ϊ0.1mol��Na2CO3�����ʵ�����Na2CO3?10H2O�����ʵ�����ȣ�Na2CO3?10H2O�����ʵ���Ϊ0.1mol��Na2CO3?10H2O������Ϊ��0.1mol��286g/mol=28.6g���ʴ�Ϊ��28.6g��
��2����ƽƽ��ԭ��Ϊ����������=����������+������ֵ������ʵ�ʳƵ�̼���ƾ��������Ϊ25g-3.6g=21.4g��
�ʴ�Ϊ���������Ʒλ�õߵ���21.4g��
II����1��ʵ������90ml����ƿ���밴100ml����ƿ���㣬����CŨ��VŨ=Cϡ��Vϡ��2.5mol/L��0.1L=
��V=13.6mL���ʴ�Ϊ��13.6��
��2�����Ʋ����м��㡢�������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ����Ͳ��ȡŨ���ᣬ��Ũ���ᵹ���ձ������ܽ⣬��ȴ��ת�Ƶ�100mL����ƿ�У����ò���������������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ����100mL��Һ��������Ҫ�������в���������ƽ��ҩ�ס��ձ�����ͷ�ιܡ�100mL����ƿ�����Ի���Ҫ����������ͷ�ιܡ�100mL����ƿ���ʴ�Ϊ������������ͷ�ιܡ�100mL����ƿ��
��3��A������Ͳ��ȡŨ����ʱ���ӣ�����Ͳ��ȡҺ��ʱ�����Ӷ�����ʹ����Һ������ƫС��ʹ���Ƶ���ҺŨ��ƫС����A����
B��ϡ������ʱ��δ��ȴ�����¼�ת�Ƶ�����ƿ�У�Һ������������������ʣ��ܽ��û����ȴ������ֱ��ת�Ƶ�����ƿ����ȴ��ᵼ��������Һ�����ƫС��������ҺŨ��ƫ�ߣ���B��ȷ��
C����ȡŨH2SO4�����Ͳ����ϴ�ӣ�����ϴ��Һת�Ƶ�����ƿ�У���Ͳ�����ʱ�Ϳ����˲�ϴ�Ӵ����������ԣ�ϴ����Ͳֻ��ʹ���Ƶ�Ũ��ƫ��C��ȷ��
D������ҡ�Ⱥ���Һ����ڿ̶��ߣ����ý�ͷ�ιܼ�����ˮ����Һ����ʹ���̶������У����ݡ�ҡ�ȡ����ú��ְ�����ڿ̶����ּ�ˮ���̶��ߣ���Һ�����ƫ��Ũ��ƫС����D����
E������ƿ���������ƿϴ�Ӻ��ڱ���ˮ���δ�����ﴦ������Һ��������䣬Ũ�Ȳ��䣬��E����
��ѡBC��
��2����ƽƽ��ԭ��Ϊ����������=����������+������ֵ������ʵ�ʳƵ�̼���ƾ��������Ϊ25g-3.6g=21.4g��
�ʴ�Ϊ���������Ʒλ�õߵ���21.4g��
II����1��ʵ������90ml����ƿ���밴100ml����ƿ���㣬����CŨ��VŨ=Cϡ��Vϡ��2.5mol/L��0.1L=
| VmL��1.84g/cm3��98% |
| 98g/mol |
��2�����Ʋ����м��㡢�������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ����Ͳ��ȡŨ���ᣬ��Ũ���ᵹ���ձ������ܽ⣬��ȴ��ת�Ƶ�100mL����ƿ�У����ò���������������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ����100mL��Һ��������Ҫ�������в���������ƽ��ҩ�ס��ձ�����ͷ�ιܡ�100mL����ƿ�����Ի���Ҫ����������ͷ�ιܡ�100mL����ƿ���ʴ�Ϊ������������ͷ�ιܡ�100mL����ƿ��
��3��A������Ͳ��ȡŨ����ʱ���ӣ�����Ͳ��ȡҺ��ʱ�����Ӷ�����ʹ����Һ������ƫС��ʹ���Ƶ���ҺŨ��ƫС����A����
B��ϡ������ʱ��δ��ȴ�����¼�ת�Ƶ�����ƿ�У�Һ������������������ʣ��ܽ��û����ȴ������ֱ��ת�Ƶ�����ƿ����ȴ��ᵼ��������Һ�����ƫС��������ҺŨ��ƫ�ߣ���B��ȷ��
C����ȡŨH2SO4�����Ͳ����ϴ�ӣ�����ϴ��Һת�Ƶ�����ƿ�У���Ͳ�����ʱ�Ϳ����˲�ϴ�Ӵ����������ԣ�ϴ����Ͳֻ��ʹ���Ƶ�Ũ��ƫ��C��ȷ��
D������ҡ�Ⱥ���Һ����ڿ̶��ߣ����ý�ͷ�ιܼ�����ˮ����Һ����ʹ���̶������У����ݡ�ҡ�ȡ����ú��ְ�����ڿ̶����ּ�ˮ���̶��ߣ���Һ�����ƫ��Ũ��ƫС����D����
E������ƿ���������ƿϴ�Ӻ��ڱ���ˮ���δ�����ﴦ������Һ��������䣬Ũ�Ȳ��䣬��E����
��ѡBC��
���������⿼����һ�����ʵ���Ũ����Һ�����ƣ�����ʵ�鲽������ǽ��Ĺؼ����Ѷ��еȣ�

��ϰ��ϵ�д�
 ��һ����ͬ���ɽ�����ϵ�д�
��һ����ͬ���ɽ�����ϵ�д� ������Ӧ���ϵ�д�
������Ӧ���ϵ�д� ��ʦ�㾦�ִʾ��ƪϵ�д�
��ʦ�㾦�ִʾ��ƪϵ�д�
�����Ŀ
��14�֣�I��ʵ������Na2CO3��10H2O��������50 g��������Ϊ21.2����Na2CO3��Һ���ش��������⣺
��1��Ӧ��������ƽ��ȡNa2CO3��10H2O���� g��
��2����������ƽ��С�ձ��Ƴ�̼���ƾ������������ƽƽ����״̬����ͼ����ͼ�п��Կ�������ͬѧ�ڲ���ʱ����һ�������� ��ʵ�ʳ�����̼���ƾ�������Ϊ g��
II. ʵ����Ҫ����2.5 mol/L ��ϡ������Һ90 mL���ش��������⣺
��1������Ͳ��ȡ��������Ϊ98�����ܶ�Ϊ1.84 g/cm3��Ũ���� mL��
��2������ʱ������ʹ�õ���������Ͳ���ձ����������⣬��ȱ�ٵ������� ��
��3��������Һ�Ĺ����У�������������ȷ�����в�����ʹ������ҺŨ��ƫ�ߵ��� ��
| A����ȡŨ����ʱ�����Ӷ��� |
| B��ϴ����ȡŨH2SO4�����Ͳ������ϴ��Һת�Ƶ�����ƿ�� |
| C��ϡ������ʱ������Һ���������� |
| D��û��ϴ��ϡ��������ձ��Ͳ����� |
F������ƿ������
��4��������ƿ��ȡ����Һ40 mL����5 mol/L��NaOH��Һ mLǡ����ȫ��Ӧ����Ӧ����Һ�е�c(Na��)�� ��������Һ��Ϲ����е�����仯��