��Ŀ����
��������������IJ���ˮ��Ƶ�����֡�ˮ����������ԭ����ˮ��ijЩӪ��Ԫ�غ������ߣ�����������������
(1)����Ϊ����������������Ӫ��Ԫ����Ҫ��____________________��
(2)��������ˮ�����������·ֽ������IJ�����
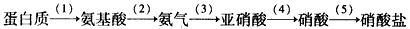
�����������У���Ԫ�صĻ��ϼ�Ϊ+3�۵���_______�������ƣ��������ӵĻ�ѧʽ��____________��д��������̼��Ʒ�Ӧ�Ļ�ѧ����ʽ____________________��
(3)����ӿ�����ȾԴ�ĽǶȣ����������ֹ��ˮ��������Ĵ�ʩ��
��_______________________����________________________��
(3)����ӿ�����ȾԴ�ĽǶȣ����������ֹ��ˮ��������Ĵ�ʩ��
��_______________________����________________________��
(1)N��P
(2)�����NH3��CaCO3+2HNO3=Ca(NO3)2+CO2��+H2O
(3)�ٽ�ֹʹ�ú���ϴ�·ۣ��ڿ��ƻ��ʵ�ʹ����
(2)�����NH3��CaCO3+2HNO3=Ca(NO3)2+CO2��+H2O
(3)�ٽ�ֹʹ�ú���ϴ�·ۣ��ڿ��ƻ��ʵ�ʹ����

��ϰ��ϵ�д�
�����Ŀ