��Ŀ����
ʵ��������Ҫ�������Ȼ�����Һ��������ֻ�л��������ơ�̼����淋��Ȼ��ƹ��壮ijѧ��������ᴿ������ͼ��
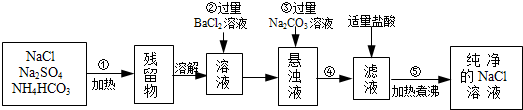
�����÷������У���ش��������⣺
��1���������ܷ�������ᱵ��Һ��______����ܡ����ܡ�����������______
______��
��2�������ڷ�����Ӧ�����ӷ���ʽΪ______��
��3�����в����ں��ж�SO42-�ѳ����ķ����ǣ�______��
��4�������۵�Ŀ����______�������ܵ�������______��
��5�������ݵ�Ŀ����______��Ҫ���ƵõĴ����Ȼ�����Һ����Ȼ��ƾ��壬�ɲ��õķ�����______��

�����÷������У���ش��������⣺
��1���������ܷ�������ᱵ��Һ��______����ܡ����ܡ�����������______
______��
��2�������ڷ�����Ӧ�����ӷ���ʽΪ______��
��3�����в����ں��ж�SO42-�ѳ����ķ����ǣ�______��
��4�������۵�Ŀ����______�������ܵ�������______��
��5�������ݵ�Ŀ����______��Ҫ���ƵõĴ����Ȼ�����Һ����Ȼ��ƾ��壬�ɲ��õķ�����______��
��1��������������Һ�������ƺ������Ʒ�Ӧ�������ᱵ�������ƣ���ȥ�������ƣ��������������Ƶ��µ����ʣ��ʴ�Ϊ�����ܣ���ʹ��Һ�������µ���������NO3-��
��2�������������������Ӧ�������ᱵ������Ba2++SO42-=BaSO4�����ʴ�Ϊ��Ba2++SO42-=BaSO4����
��3����������Ȼ�����Һ��ȥ��������ӣ���������������ѳ������ɾ�ֹƬ�����ϲ���Һ�����μ�һ���Ȼ�����Һ�������ֻ��Ǿ�˵������������Ѿ��������ʴ�Ϊ����Һ���ú�ȡ�����ϲ���Һ�������Һ���ˣ�ȡ������Һ���μ�����BaCl2��Һ�����ް�ɫ�������ɣ�����Һ�е�SO42-�Ѿ�������
��4������̼������Һ����ȥ�������Ȼ��������˳���̼�ᱵ���ʴ�Ϊ����ȥ��Һ�й�����Ba2+�����ˣ�
��5�����������Һ��Ŀ���dz�ȥ��Һ���ܽ��CO2�Ͷ�������ᣬ����Ũ���ᾧ�ɵõ��Ȼ��ƾ��壬�ʴ�Ϊ����ȥ������HCl���ܽ�����Һ�е�CO2������Ũ���ᾧ��
��2�������������������Ӧ�������ᱵ������Ba2++SO42-=BaSO4�����ʴ�Ϊ��Ba2++SO42-=BaSO4����
��3����������Ȼ�����Һ��ȥ��������ӣ���������������ѳ������ɾ�ֹƬ�����ϲ���Һ�����μ�һ���Ȼ�����Һ�������ֻ��Ǿ�˵������������Ѿ��������ʴ�Ϊ����Һ���ú�ȡ�����ϲ���Һ�������Һ���ˣ�ȡ������Һ���μ�����BaCl2��Һ�����ް�ɫ�������ɣ�����Һ�е�SO42-�Ѿ�������
��4������̼������Һ����ȥ�������Ȼ��������˳���̼�ᱵ���ʴ�Ϊ����ȥ��Һ�й�����Ba2+�����ˣ�
��5�����������Һ��Ŀ���dz�ȥ��Һ���ܽ��CO2�Ͷ�������ᣬ����Ũ���ᾧ�ɵõ��Ȼ��ƾ��壬�ʴ�Ϊ����ȥ������HCl���ܽ�����Һ�е�CO2������Ũ���ᾧ��

��ϰ��ϵ�д�
 �ƸԴ��ž�ϵ�д�
�ƸԴ��ž�ϵ�д�
�����Ŀ