��Ŀ����
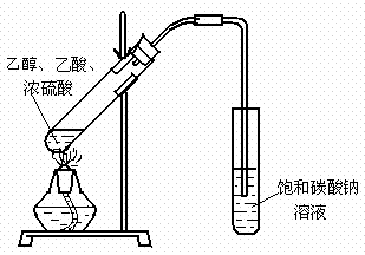
��1��ijʵ��С������ͼװ����ȡ������������ش��������⣺�ٸ�װ����һ��������ָ������ ��
��˵���ô����������ĺ���� ��
����֤����ʵ���������ʣ��������������������Ժ�ֱ�ӵ������� ��
����ɸ÷�Ӧ�Ļ�ѧ����ʽ
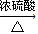 ���������б��18O��λ�ã�
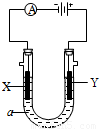
���������б��18O��λ�ã���2�����ԭ���ڻ�ѧ��ҵ���й㷺Ӧ�ã���ͼ��ʾһ�����أ�װ�е��Һa��a��CuCl2��Һ��X��Y����ʯī�缫��ͨ��������ֱ����Դ������
��X���� ������������������������X��������
��Y���ϵĵ缫��Ӧʽ ��
������Ӧ��������Y���ϲ�������224mL����״��������˹����е�Դ���ṩ�ĵ����� ������֪ÿ�����Ӵ��ĵ���ΪQ���ú�Q��ʽ�ӱ�ʾ��
���𰸡���������1���ٷ�Ӧ�Թ����Ȳ�����ʢ����̼������Һ���Թ��еĵ�������Һ���¿��ܷ���������
���Ҵ������ᶼ������ˮ����������������ˮ����Һ�ֲ㣬�ܶȱ�ˮС�������������ϲ㣻
�������봼������������Ӧ�У������е��Ȼ��ṩ-OH�����е�-OH�ṩ-H����������ˮ��ʣ����Ž����������������
��2���ٵ������ӵ�Դ�����ĵ缫�������������ų���ԭ��Ӧ���������������ŵ磬��Һ��Cu2+��������ǿ����Cu2+�ŵ�
����Cu��
��Y�����ӵ�Դ���������ǵ��ص�����������������Ӧ��Cl-�������ŵ�����Cl2��
�ۼ��ϲ���224mL�������������������������ʵ��������ݵ���ת�Ƽ���ת�Ƶĵ��ӵ����ʵ��������������Դ���ṩ�ĵ�����
����⣺��1���ٷ�Ӧ�Թ����Ȳ�����ʢ����̼������Һ���Թ��еĵ�������Һ���¿��ܷ���������
�ʴ�Ϊ����������̼������ҺҺ���£����ܷ���������
���Ҵ������ᶼ������ˮ����������������ˮ����Һ�ֲ㣬�ܶȱ�ˮС�������������ϲ㣬����̼������ҺҺ���ϳ���һ����ɫ��״Һ�壬˵�������������ɣ�
�ʴ�Ϊ������̼������ҺҺ���ϳ���һ����ɫ��״Һ�壻
�������봼������������Ӧ�У������е��Ȼ��ṩ-OH�����е�-OH�ṩ-H����������ˮ��ʣ����Ž����������������ͬʱ�÷�Ӧ���棬��Ӧ�Ļ�ѧ����ʽΪCH3CH218OH+CH3COOH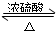 CH3CO18OCH2CH3+H2O��
CH3CO18OCH2CH3+H2O��
�ʴ�Ϊ��CH3CH218OH+CH3COOH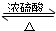 CH3CO18OCH2CH3+H2O��
CH3CO18OCH2CH3+H2O��
��2���ٵ������ӵ�Դ�����ĵ缫�������������ų���ԭ��Ӧ���������������ŵ磬��Һ��Cu2+��������ǿ����Cu2+�ŵ�
������ӦCu2++2e-=Cu����X��������������ɫ���壻
�ʴ�Ϊ������������ɫ���壻
��Y�����ӵ�Դ���������ǵ��ص�����������������Ӧ��Cl-�������ŵ�����Cl2���缫��ӦʽΪ2Cl--2e-=Cl2����
�ʴ�Ϊ��2Cl--2e-=Cl2����
�ۼ��ϲ���224mL���������������������ʵ���Ϊ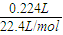 =0.01mol�����ݵ���ת���غ��֪ת�Ƶĵ��ӵ����ʵ���Ϊ0.01mol×2=0.02mol���ʵ�Դ���ṩ�ĵ���Ϊ0.02mol×6.02×1023mol-1×Q=1.204×1022Q��
=0.01mol�����ݵ���ת���غ��֪ת�Ƶĵ��ӵ����ʵ���Ϊ0.01mol×2=0.02mol���ʵ�Դ���ṩ�ĵ���Ϊ0.02mol×6.02×1023mol-1×Q=1.204×1022Q��
�ʴ�Ϊ��1.204×1022Q��
���������⿼�������������Ʊ������ع���ԭ���ȣ��ѶȲ���ע����������ʵ����Һ�����ơ�����̼������Һ�������Լ�������Ӧ�Ļ�����
���Ҵ������ᶼ������ˮ����������������ˮ����Һ�ֲ㣬�ܶȱ�ˮС�������������ϲ㣻
�������봼������������Ӧ�У������е��Ȼ��ṩ-OH�����е�-OH�ṩ-H����������ˮ��ʣ����Ž����������������
��2���ٵ������ӵ�Դ�����ĵ缫�������������ų���ԭ��Ӧ���������������ŵ磬��Һ��Cu2+��������ǿ����Cu2+�ŵ�
����Cu��
��Y�����ӵ�Դ���������ǵ��ص�����������������Ӧ��Cl-�������ŵ�����Cl2��
�ۼ��ϲ���224mL�������������������������ʵ��������ݵ���ת�Ƽ���ת�Ƶĵ��ӵ����ʵ��������������Դ���ṩ�ĵ�����
����⣺��1���ٷ�Ӧ�Թ����Ȳ�����ʢ����̼������Һ���Թ��еĵ�������Һ���¿��ܷ���������
�ʴ�Ϊ����������̼������ҺҺ���£����ܷ���������
���Ҵ������ᶼ������ˮ����������������ˮ����Һ�ֲ㣬�ܶȱ�ˮС�������������ϲ㣬����̼������ҺҺ���ϳ���һ����ɫ��״Һ�壬˵�������������ɣ�
�ʴ�Ϊ������̼������ҺҺ���ϳ���һ����ɫ��״Һ�壻
�������봼������������Ӧ�У������е��Ȼ��ṩ-OH�����е�-OH�ṩ-H����������ˮ��ʣ����Ž����������������ͬʱ�÷�Ӧ���棬��Ӧ�Ļ�ѧ����ʽΪCH3CH218OH+CH3COOH
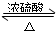 CH3CO18OCH2CH3+H2O��
CH3CO18OCH2CH3+H2O���ʴ�Ϊ��CH3CH218OH+CH3COOH
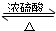 CH3CO18OCH2CH3+H2O��
CH3CO18OCH2CH3+H2O����2���ٵ������ӵ�Դ�����ĵ缫�������������ų���ԭ��Ӧ���������������ŵ磬��Һ��Cu2+��������ǿ����Cu2+�ŵ�
������ӦCu2++2e-=Cu����X��������������ɫ���壻
�ʴ�Ϊ������������ɫ���壻
��Y�����ӵ�Դ���������ǵ��ص�����������������Ӧ��Cl-�������ŵ�����Cl2���缫��ӦʽΪ2Cl--2e-=Cl2����
�ʴ�Ϊ��2Cl--2e-=Cl2����
�ۼ��ϲ���224mL���������������������ʵ���Ϊ
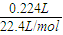 =0.01mol�����ݵ���ת���غ��֪ת�Ƶĵ��ӵ����ʵ���Ϊ0.01mol×2=0.02mol���ʵ�Դ���ṩ�ĵ���Ϊ0.02mol×6.02×1023mol-1×Q=1.204×1022Q��
=0.01mol�����ݵ���ת���غ��֪ת�Ƶĵ��ӵ����ʵ���Ϊ0.01mol×2=0.02mol���ʵ�Դ���ṩ�ĵ���Ϊ0.02mol×6.02×1023mol-1×Q=1.204×1022Q���ʴ�Ϊ��1.204×1022Q��
���������⿼�������������Ʊ������ع���ԭ���ȣ��ѶȲ���ע����������ʵ����Һ�����ơ�����̼������Һ�������Լ�������Ӧ�Ļ�����

��ϰ��ϵ�д�
�����Ŀ

 ��1��ijʵ��С������ͼװ����ȡ������������ش��������⣺
��1��ijʵ��С������ͼװ����ȡ������������ش��������⣺
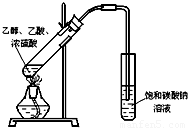
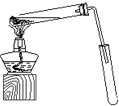 ij��ѧʵ��С������ͼ��ʾ��װ����ȡ������������������ �� �� �����Ƿ����������ʣ�����̨�����ӵ�֧������ʡ�ԣ�����֪���������ķе�Ϊ77.1�棬�Ҵ��е�Ϊ78.4�棬����ķе�Ϊ118�森�����Ҫ����գ�
ij��ѧʵ��С������ͼ��ʾ��װ����ȡ������������������ �� �� �����Ƿ����������ʣ�����̨�����ӵ�֧������ʡ�ԣ�����֪���������ķе�Ϊ77.1�棬�Ҵ��е�Ϊ78.4�棬����ķе�Ϊ118�森�����Ҫ����գ�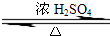 CH3COOCH2CH3+H2O
CH3COOCH2CH3+H2O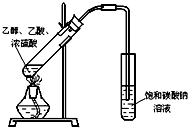
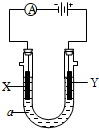
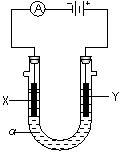 ��2�����ԭ���ڻ�ѧ��ҵ���й㷺Ӧ�á���ͼ��ʾһ�����أ�װ�е��Һa��a��CuCl2��Һ��X��Y����ʯī�缫��ͨ��������ֱ����Դ������
��2�����ԭ���ڻ�ѧ��ҵ���й㷺Ӧ�á���ͼ��ʾһ�����أ�װ�е��Һa��a��CuCl2��Һ��X��Y����ʯī�缫��ͨ��������ֱ����Դ������