��Ŀ����
����˵������ȷ���ǣ� ��A����Na2CO3��Һ��ͨ�������CO2����Һ������������
B����8gSO3����92gˮ�У�������Һ������������Ϊ8%
C����֪H+��aq��+OH-��aq��=H2O��l������H=-57.3kJ/mol����4g�������ƹ������100mL1mol/L��ϡ�����У��ų���5.73kJ������
D����100ml1mol/L��Ca��HCO3��2��Һ�м����Ũ�ȵ������NaOH��Һ����Һ�ļ��Լ���
���𰸡�������A����Na2CO3��Һ��ͨ�������CO2����Һ����ǣ�
B��SO3��ˮ��Ӧ����H2SO4��
C���������ƹ�������ˮ���ȣ�
D����100ml1mol/L��Ca��HCO3��2��Һ�м����Ũ�ȵ������NaOH��Һ������CaCO3��NaHCO3��
����⣺A����Na2CO3��Һ��ͨ�������CO2������NaHCO3������NaHCO3���ܽ�ȱ�Na2CO3��С������ҺӦ����ǣ���A����
B��SO3��ˮ��Ӧ����H2SO4��������Һ����ΪH2SO4����������Ϊ9.6%����B����
C���������ƹ�������ˮ���ȣ���4g�������ƹ������100mL1mol/L��ϡ�����У��ų�����������5.73kJ����C����
D����100ml1mol/L��Ca��HCO3��2��Һ�м����Ũ�ȵ������NaOH��Һ��������Ca��HCO3��2+NaOH=CaCO3��+NaHCO3+H2O����ҺHCO3-Ũ�ȼ�С��ˮ������OH-��Ũ�ȼ�С����Һ�ļ��Լ�������D��ȷ��
��ѡD��
���������⿼���Ϊ�ۺϣ��漰����ˮ���Ӧ�á��к����Լ����ӷ�Ӧ�����⣬��Ŀ�Ѷ��еȣ�����ע���NaHCO3���ܽ�ȱ�Na2CO3��С�����������ƹ�������ˮ���ȣ�
B��SO3��ˮ��Ӧ����H2SO4��
C���������ƹ�������ˮ���ȣ�
D����100ml1mol/L��Ca��HCO3��2��Һ�м����Ũ�ȵ������NaOH��Һ������CaCO3��NaHCO3��
����⣺A����Na2CO3��Һ��ͨ�������CO2������NaHCO3������NaHCO3���ܽ�ȱ�Na2CO3��С������ҺӦ����ǣ���A����
B��SO3��ˮ��Ӧ����H2SO4��������Һ����ΪH2SO4����������Ϊ9.6%����B����
C���������ƹ�������ˮ���ȣ���4g�������ƹ������100mL1mol/L��ϡ�����У��ų�����������5.73kJ����C����
D����100ml1mol/L��Ca��HCO3��2��Һ�м����Ũ�ȵ������NaOH��Һ��������Ca��HCO3��2+NaOH=CaCO3��+NaHCO3+H2O����ҺHCO3-Ũ�ȼ�С��ˮ������OH-��Ũ�ȼ�С����Һ�ļ��Լ�������D��ȷ��
��ѡD��
���������⿼���Ϊ�ۺϣ��漰����ˮ���Ӧ�á��к����Լ����ӷ�Ӧ�����⣬��Ŀ�Ѷ��еȣ�����ע���NaHCO3���ܽ�ȱ�Na2CO3��С�����������ƹ�������ˮ���ȣ�

��ϰ��ϵ�д�
�����Ŀ
����˵������ȷ���ǣ�������
| A�������Ƶ��й�����ʵ��ʱ����ʣ����Ӧ�Ż�ԭ�Լ�ƿ | B������25.00 ml ��ʽ�ζ���ȷ��ȡ20.00 ml KMnO4��Һ | C����ʪ��ĵ��۵⻯����ֽ���Լ���NO2��Br2���� | D��ij��Һ�м��������ܲ���ʹ����ʯ��ˮ����ǵ����壬�����Һ��һ������CO32- |
Na3N��NaH�������ӻ������ˮ��Ӧ�����������ɣ�����˵������ȷ���ǣ�������
| A���������ʵ������Ӱ뾶���������Ӱ뾶С | B������ˮ��������Һ����ʹ��ɫ��̪��� | C����ˮ��Ӧʱ��ˮ���������� | D�������ᷴӦ��ֻ����һ���� |
����˵������ȷ���ǣ�������
| A���ü�ʯ�ҳ�ȥ�����е�ˮ | B���⻯������Ҫ�ĸй���ϣ��廯���������˹����� | C���������ƿ�����DZͧ����������Դ | D����ɫ��Ӧʵ������������ϴ��˿�����պ���մȡ�����ھƾ��������չ۲� |
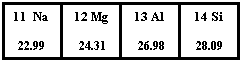 ��ͼΪԪ�����ڱ��������ڵ�һ���֣��ݴ��ж�����˵������ȷ���ǣ�������
��ͼΪԪ�����ڱ��������ڵ�һ���֣��ݴ��ж�����˵������ȷ���ǣ�������| A��SiԪ�ص������ǹ裬��˵����Ϊ14 | B��Mgԭ�ӵĽṹʾ��ͼ��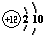 | C��Al�����ԭ��������26.98g | D�����Ƕ����ڽ���Ԫ�� |
����˵������ȷ���ǣ�������
| A��O��Na��SԪ�ص�ԭ�Ӱ뾶�������� | B��KOH��Mg��OH��2��Ba��OH��2�ļ���������ǿ | C��H2SO4��H3PO4��HClO4������������ǿ | D��F2��Cl2��Br2��I2�����������μ��� |