��Ŀ����
�������һ����Ҫ�ķǽ������ϡ��Ʊ��������Ҫ�������£�
�ٸ�������̼��ԭ���������Ƶôֹ裻
�ڴֹ������HCl���巴Ӧ�Ƶ�SiHCl3��Si��3HCl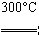 SiHCl3��H2��
SiHCl3��H2��
��SiHCl3�����H2��1000��1100��C��Ӧ�Ƶô��衣
��֪SiHCl3����H2O���ҷ�Ӧ���ڿ���������ȼ��
��ش��������⣺
(1)�ڢ��Ʊ��ֹ�Ļ�ѧ����ʽΪ__________________________ ____________________________________________________________________________________________________________________��
(2)�ֹ���HCl��Ӧ��ȫ�������õ���SiHCl3(�е�33.0��C)�к�������SiCl4(�е�57.6��C)��HCl(�е�84.7��C)���ᴿSiHCl3�ķ���Ϊ__________________________________��
(3)��SiHCl3�����H2��Ӧ�Ʊ������װ����ͼ��ʾ(��Դ���г�װ�þ�����ȥ)��
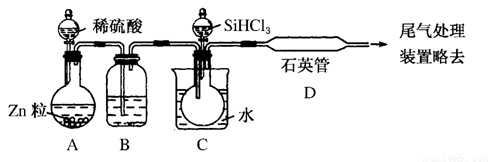
��װ��B�е��Լ���________��װ��C�е���ƿ��Ҫ���ȣ���Ŀ����__________________��
��װ��D�з�����Ӧ�Ļ�ѧ����ʽΪ__________________________________________________________________________________________________________________________��
��Ϊ��֤�Ʊ�����ʵ��ijɹ��������Ĺؼ��Ǽ��ʵ��װ�õ������ԣ����ƺ÷�Ӧ�¶��Լ�______________________________��
(1)SiO2��2C Si��2CO��
Si��2CO��
(2)����(������)
(3)��Ũ���ᡡʹ������ƿ�е�SiHCl3����
��SiHCl3��H2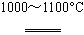 Si��3HCl�����ž�װ���еĿ���
Si��3HCl�����ž�װ���еĿ���
��������(1)��������Ϣ��������̿��ԭ���������Ƶôֹ裬��д����Ӧ����ʽSiO2��2C Si��2CO����
Si��2CO����
(2)������������������и��е㣬SiHCl3�е���ͣ��ɲ��÷���(������)�ķ�������SiHCl3���������
(3)SiHCl3����ˮ���ҷ�Ӧ��Ӧ���Ƶõ�H2�е�ˮ������ȥ����װ��B�е��Լ���Ũ���ᡣ������SiHCl3ΪҺ�壬��ӦʱӦʹ������������D����H2��Ӧ����װ��C����ƿ����ȡ��Ʊ������ʵ��ɹ����������������Ӧ�ž���ϵ�еĿ�������ΪSiHCl3�ڿ���������ȼ��H2�ڼ���ʱ�������Ӧ��������ը��

��1��̫������ˮ���г�ʹ��һ�����������Ͻ������Ϊ���ռ���̫��������Ϳ�㣮
����д����̬��ԭ�ӵĺ�������Ų�ʽ______��
��NiO��FeO�ľ���ṹ���;����Ȼ�����ͬ��Ni2+��Fe2+�����Ӱ뾶�ֱ�Ϊ69pm��78pm�����۵�NiO______�������������FeO��
��Ni��Fe��Co�Ƚ���������CO��Ӧ�γ�����Fe��CO��5�����³�Һ̬���۵�Ϊ-20.5�棬�е�Ϊ103�棬�����ڷǼ����ܼ����ݴ˿��ж�Fe��CO��5��������______��������ͣ�����λ����______��
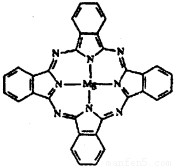
��2������̪ݼ������ڹ�̫���ܵ��������Ҫ���ã�һ�ֽ���þ̪ݼ�����Ľṹ����ͼ��������ͼ���ü�ͷ��ʾ����λ����
��3��CO��N2��Ϊ�ȵ����壮CO���ܼ��ܴ���N2���ܼ��ܣ���CO��N2���ײμӻ�ѧ��Ӧ�������±����ݣ�˵��CO��N2���õ�ԭ����______��
| A-B | A=B | A��B | ||
| CO | ���� | 357.7 | 798.9 | 1071.9 |
| ���ܲ�ֵ | 441.2 273 | |||
| N2 | ���� | 154.8 | 418.3 | 941.7 |
| ���ܲ�ֵ | 263.6 523.3 | |||
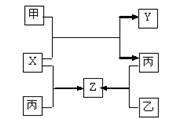
�ס��ҡ��������ֳ������ʣ�X��Y��Z�dz������������֮��������ת����ϵ��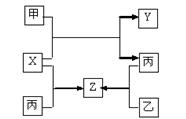
��֪���Ƕ����ڽ������ʣ��ҡ����Ƕ����ڷǽ������ʣ�X��Y��Z��ֻ��һ�������Ӿ��塣����˵������ȷ����
| A��X�Ǿ��м��Լ��ķǼ��Է��� | B��Z��ˮú������Ҫ�ɷ�֮һ |
| C����X�ķ�Ӧ�����ȷ�Ӧ | D���������������Ҫԭ�� |
 ��Դ���������Ϊ��Լ������ᾭ�÷�չ��ƿ����Խ��Խ��Ĺ��ҿ�ʼʵ�С�����ƻ���������̫������Դ��Ѱ�÷�չ���¶�����
��Դ���������Ϊ��Լ������ᾭ�÷�չ��ƿ����Խ��Խ��Ĺ��ҿ�ʼʵ�С�����ƻ���������̫������Դ��Ѱ�÷�չ���¶�����