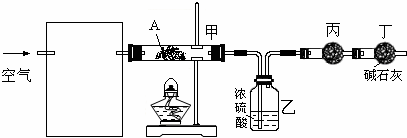
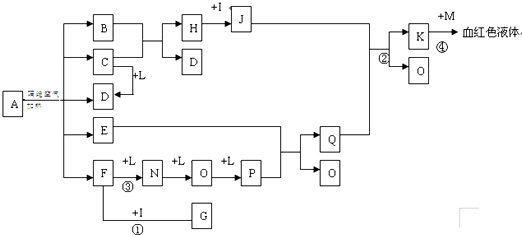
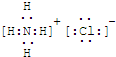��Ŀ����
��֪������EΪ��ɫ��ζ��Һ�壬FΪ����ɫ��ĩ��GΪ��������ɫ����(��Ӧ����������)����ش��������⣺
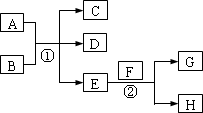
(1)��д��F�ĵ���ʽ________���ڢڷ�Ӧ�У�������2.24 L��G(��״��)ʱ����Ӧת�Ƶĵ�����ĿΪ________
(2)��A��C��D��������Ԫ�أ���A�Ļ��ϼ۽���C��D֮�䣬д��ϡ��Һ�Тٵ����ӷ���ʽ��________��
(3)��C��D��Ϊ�����Ҷ���ʹ����ʯ��ˮ����ǣ���A��B�ֱ�Ϊ________��(д��ѧʽ)
(4)��A��B��Ϊ���廯���C�dz�����һ�����Ը��������Ӧ�ٵĻ�ѧ����ʽΪ________����1.7 g��D��O2��Ӧ������̬�IJ���ʱ�ų�22.67 kJ������д���÷�Ӧ���Ȼ�ѧ����ʽ��________��
�𰸣�
������
������
|
����(1) ����(2)Cl2��2OH�� ����(3)C��ŨH2SO4��(Ũ��ûд��1��)(3��) ����(4)2NH4Cl��Ca(OH)2 ����4NH3(g)��5O2(g)��4NO(g)��6H2O(g)����H����906.8 KJ/mol(2��) |

��ϰ��ϵ�д�
�����Ŀ

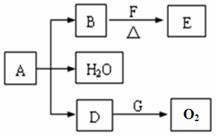
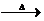 CH3CHO+H2O+Cu
CH3CHO+H2O+Cu