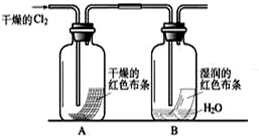
��Ŀ����
��16�֣�ij��ѧ��ȤС��Ϊ��̽��������ij�ǽ����������γɵ�δ֪����ijɷ֡���С���Ա������ͨ�����ʯ��ˮ�����ֳ���ʯ��ˮ����ǣ�����ͨ�뷢�ֻ����ֱ���壬�ɴ˸�С���Ա������ijɷ�������롣
��������롿��
����1��______________________________��
����2��______________________________��
����3��______________________________��
Ϊ����֤���룬��С�����ʵ�����̽����
��ʵ��̽����
��С��ͬѧ����ͼ��ʾװ�ã��������a��ͨ�룬��
(1) B�п���װ����________�Լ�(����)��
A��NaCl��Һ�� B��KMnO4��Һ C������
D������ʯ��ˮ E��NaHCO3 ��Һ F����ˮ
(2) A��Ʒ����Һ�������ǣ�_________________________________��
(3) D�г���ʯ��ˮ�������ǣ�_________________________________��
ͨ����ʵ�飬��С��ͬѧ�۲쵽��������ʵ������
��A��Ʒ����Һ��ɫ����C��Ʒ����Һ����ɫ ��D�г���ʯ��ˮ�����
���ó����ۡ�
(4) �����������С��ͬѧȷ�ϸ�����ijɷ�Ϊ��______________________��
(5) ��д��SO2����ˮ������Ӧ�����ӷ���ʽ��_________________________��
[�������]����ΪCO2������ΪSO2������ΪCO2��SO2�Ļ������
[ʵ��̽��]�� (1)BF��ȫ�Ը��֣���
(2)֤������������Ƿ���SO2(3)֤������������Ƿ���CO2
[����]��
(4)����ΪCO2��SO2�Ļ������ (5)Cl2��SO2��2H2O===4H����2Cl�C��SO42��
����:����ʯ��ˮ����ǵ�������CO2��SO2��
��1������SO2һ����Ʒ����Һ����SO2�Ĵ��ڻ����CO2�ļ��飬�����ڼ���CO2֮ǰ��Ҫ��SO2ȫ����ȥ������������CO2��Ҳ����CO2������ѡ�õ��Լ���KMnO4��Һ������SO2���͵�ˮ����ԭSO2����ѡ��A��C���ܳ�ȥSO2��BҲ������CO2��D�����CO2��
��2��Ʒ������������SO2�ġ�
��3��ʯ��ˮ����������CO2�ġ�
��4�������������֤�������м���SO2��Ҳ��CO2��
��5����ˮ���������� ������SO2�������ᡣ

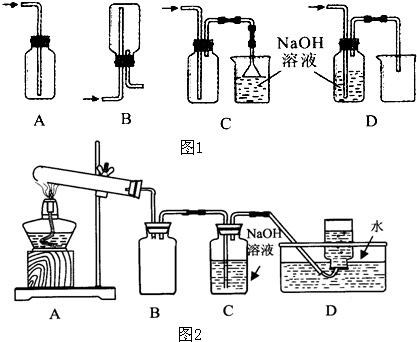
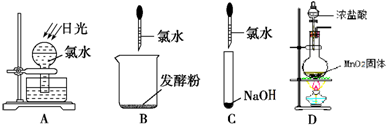
��1����ʵ��������Ũ������MnO2������ȡCl2���������ʵ�飬��ͼ1��ʾ��
��д���÷�Ӧ�����ӷ���ʽ
�������ռ�Cl2��װ����ȷװ����
�۽�Cl2ͨ��ˮ�У�������Һ�о��������Եĺ���������
�����ʵ��Ƚ�Cl2��Br2�������ԣ������������ǣ�ȡ����������ˮ��CCl4���Թ��У�
��2��ij��ѧ��ȤС��Ϊ��̽��AgNO3�����ȶ��ԣ����������ʵ�飮
����ͼ2��ʾ��ʵ��װ��A����AgNO3���壬��������ɫ���壬��װ��D���ռ�����ɫ���壮����Ӧ�����Ժ��Թ��в�������Ϊ��ɫ��
��װ��B��������
�ھ�С�����۲���֤����ɫ����ΪO2������֤������
���������ϡ�Ag2O�ͷ�ĩ��Ag��Ϊ��ɫ��Ag2O�����ڰ�ˮ��
��������衿�Թ��в����ĺ�ɫ��������ǣ���Ag����Ag2O����Ag��Ag2O
��ʵ����֤����С��Ϊ��֤�������룬�ֱ�ȡ������ɫ���壬����������ʵ�飮
| ʵ���� | ���� | ���� |
| a | ����������ˮ���� | ��ɫ���岻�ܽ� |
| b | ��������ϡ���ᣬ�� | ��ɫ�����ܽ⣬����������� |
��ʵ����ۡ���������ʵ�������ܸ�С��ó���AgNO3�����ȷֽ�Ļ�ѧ����ʽΪ
 ij��ѧ��ȤС��Ϊ��̽���ڳ�����ij�ǽ����������γɵ�δ֪����ijɷ֣���С���Ա������ͨ�����ʯ��ˮ�����ֱ���ǣ�����ͨ�뷢�ֻ����ֱ���壬�ɴ˸�С���Ա������ijɷ�������룮
ij��ѧ��ȤС��Ϊ��̽���ڳ�����ij�ǽ����������γɵ�δ֪����ijɷ֣���С���Ա������ͨ�����ʯ��ˮ�����ֱ���ǣ�����ͨ�뷢�ֻ����ֱ���壬�ɴ˸�С���Ա������ijɷ�������룮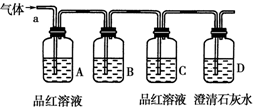 ij��ѧ��ȤС��Ϊ��̽��������ij�ǽ����������γɵ�δ֪����ijɷ֣���С���Ա������ͨ�����ʯ��ˮ�����ֳ���ʯ��ˮ����ǣ�����ͨ�뷢�ֻ����ֱ���壬�ɴ˸�С���Ա������ijɷ�������룮
ij��ѧ��ȤС��Ϊ��̽��������ij�ǽ����������γɵ�δ֪����ijɷ֣���С���Ա������ͨ�����ʯ��ˮ�����ֳ���ʯ��ˮ����ǣ�����ͨ�뷢�ֻ����ֱ���壬�ɴ˸�С���Ա������ijɷ�������룮