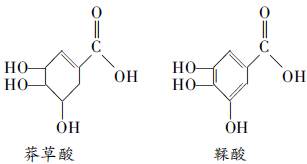
��Ŀ����
1,3�D����������Ҫ�Ļ���ԭ�ϣ�����ϩ�ϳ�1,3�D��������·�����£�
CH2=CH2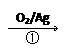
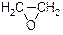
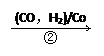 HOCH2CH2CHO
HOCH2CH2CHO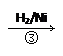 HOCH2CH2CH2OH
HOCH2CH2CH2OH
��ͨ����Ӧ������ϩ�Ϳ�����������Ʊ�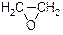 ����÷�Ӧǰ��ijһʱ�����������������±���
����÷�Ӧǰ��ijһʱ�����������������±���
�����ʱ��ϩ��ת���ʡ�
��ij�������ѹ�����ϩ14t�����ǵ�ԭ�ϵij�����ã���Ӧ�ڡ��������CO��H2��������������Ӧ��ã�
C+H2O CO+H2 CH4+H2O
CO+H2 CH4+H2O CO+3H2
CO+3H2
���������������У���Ӧ����CO��H2����Ӧ����H2������20%���ҷ�Ӧ�١��ڡ����и��л����ת���ʾ�Ϊ100%��
����������Ҫ��̿����������ٶ֣���������������Ҫ��
CH2=CH2
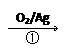
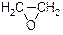
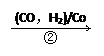 HOCH2CH2CHO
HOCH2CH2CHO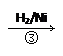 HOCH2CH2CH2OH
HOCH2CH2CH2OH��ͨ����Ӧ������ϩ�Ϳ�����������Ʊ�
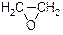 ����÷�Ӧǰ��ijһʱ�����������������±���
����÷�Ӧǰ��ijһʱ�����������������±���| | C2H4 | O2 | N2 |  |
| ��Ӧǰ������� | 25.0% | 15.0% | 60.0% | 0 |
| ijһʱ��������� | 5.56% | 5.54% | 66.7% | 22.2% |
��ij�������ѹ�����ϩ14t�����ǵ�ԭ�ϵij�����ã���Ӧ�ڡ��������CO��H2��������������Ӧ��ã�
C+H2O
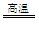 CO+H2 CH4+H2O
CO+H2 CH4+H2O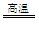 CO+3H2
CO+3H2���������������У���Ӧ����CO��H2����Ӧ����H2������20%���ҷ�Ӧ�١��ڡ����и��л����ת���ʾ�Ϊ100%��
����������Ҫ��̿����������ٶ֣���������������Ҫ��
��1��80%��2����̿3.6t������4.8t��
���跴Ӧǰ���干��100L
��ӦǰV(C2H4)=100L��25.0%=25L��V(N2)=100L��60.0%=60L
��ΪN2������������仯�����ʱ��������Ϊ60L��66.7%=90L
V(C2H4)=90L��5.56%=5.00L
���ʱ��ϩ��ת����=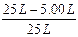 ��100%="80.0% "
��100%="80.0% "
Ҳ������������ϩת���ʣ�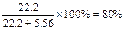
�Ʒ�Ӧ���� C2H4 ~ CO ~ H2 ~ HOCH2CH2CHO
28 28 2 74
14t m(CO)��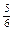 m(H2)��
m(H2)��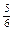 m(HOCH2CH2CHO)
m(HOCH2CH2CHO)
��m(CO)=16.8 t m(H2)="1.2t " m(HOCH2CH2CHO) ="37" t
��Ӧ���� HOCH2CH2CHO �� H2
74 2
37t m(H2)��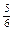
��m(H2)="1.2t "
��Ӧ�ڡ����й���CO16.8 t��H22.4t��
C + H2O CO + H2 CH4 + H2O
CO + H2 CH4 + H2O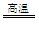 CO + 3H2
CO + 3H2
12 28 2 16 28 6
m(C) m(CH4)
�÷�����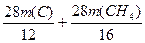 =16.8 t ��
=16.8 t �� 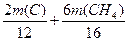 =2.4t
=2.4t
���m(C)="3.6t " m(CH4)="4.8t"
��ӦǰV(C2H4)=100L��25.0%=25L��V(N2)=100L��60.0%=60L
��ΪN2������������仯�����ʱ��������Ϊ60L��66.7%=90L
V(C2H4)=90L��5.56%=5.00L
���ʱ��ϩ��ת����=
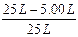 ��100%="80.0% "
��100%="80.0% " Ҳ������������ϩת���ʣ�
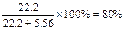
�Ʒ�Ӧ���� C2H4 ~ CO ~ H2 ~ HOCH2CH2CHO
28 28 2 74
14t m(CO)��
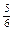 m(H2)��
m(H2)��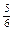 m(HOCH2CH2CHO)
m(HOCH2CH2CHO)��m(CO)=16.8 t m(H2)="1.2t " m(HOCH2CH2CHO) ="37" t
��Ӧ���� HOCH2CH2CHO �� H2
74 2
37t m(H2)��
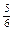
��m(H2)="1.2t "
��Ӧ�ڡ����й���CO16.8 t��H22.4t��
C + H2O
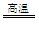 CO + H2 CH4 + H2O
CO + H2 CH4 + H2O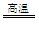 CO + 3H2
CO + 3H212 28 2 16 28 6
m(C) m(CH4)
�÷�����
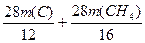 =16.8 t ��
=16.8 t �� 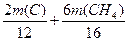 =2.4t
=2.4t���m(C)="3.6t " m(CH4)="4.8t"

��ϰ��ϵ�д�
 ��һ������ĩ�ٷֳ�̾�ϵ�д�
��һ������ĩ�ٷֳ�̾�ϵ�д�
�����Ŀ
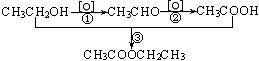
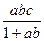
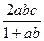
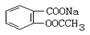 ת��Ϊ
ת��Ϊ �ķ���Ϊ�� ��
�ķ���Ϊ�� ��