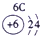
��Ŀ����
�±�ΪԪ�����ڱ���һ���֣����е���ĸ������Ӧ��Ԫ�أ�
��1��Y�ĸ�����Y-�ĺ�������Ų�ʽΪ
��2��C�Ļ�̬ԭ�Ӻ�������Ų�ʽΪ
��3��B�Ļ�̬ԭ���к������ռ��
��4��B��C��D����Ԫ�صĵ縺���ɴ�С��˳����
��5��X��Dԭ�ӽ���γɵ�XD42-���ӵ�����ԭ���ӻ�����Ϊ
��6��WԪ�صĻ�̬ԭ�Ӽ۵����Ų�Ϊ
��7��������EA��ˮ��Ӧʱ�����������ɣ��û�ѧ��Ӧ����ʽΪ
��8��������B3C4�����У�Ԫ��B�Ļ��ϼ�Ϊ
��9��Ԫ��X��Z��B��C���γɵ�Z��XBC��3������������ԭ������λ������Ҫ����1��1�ĸ������������������Ѫ��ɫ����������������ӻ�ѧʽΪ
A�����Ӽ� B�����Լ� C���Ǽ��Լ� D����λ����
| A | |||||||||||||||||
| B | C | D | |||||||||||||||
| E | X | Y | |||||||||||||||
| Z | W |
1s22s22p63s23p6
1s22s22p63s23p6
����Ԫ����Ԫ�����ڱ��е�λ��Ϊ����
��
���ڵ�����A
����A
����2��C�Ļ�̬ԭ�Ӻ�������Ų�ʽΪ
1s22s22p3
1s22s22p3
����3��B�Ļ�̬ԭ���к������ռ��
4
4
��������ͬ��ԭ�ӹ������4��B��C��D����Ԫ�صĵ縺���ɴ�С��˳����
O��N��C
O��N��C
����Ԫ�ط��ű�ʾ������5��X��Dԭ�ӽ���γɵ�XD42-���ӵ�����ԭ���ӻ�����Ϊ
sp3
sp3
�ӻ����ռ乹��Ϊ��������
��������
����6��WԪ�صĻ�̬ԭ�Ӽ۵����Ų�Ϊ
3d104s1
3d104s1
��λ��Ԫ�����ڱ�����
��
���ڵ���B
��B
�壻��7��������EA��ˮ��Ӧʱ�����������ɣ��û�ѧ��Ӧ����ʽΪ
NaH+H2O�TNaOH+H2��
NaH+H2O�TNaOH+H2��
��8��������B3C4�����У�Ԫ��B�Ļ��ϼ�Ϊ
+4
+4
���侧��ṹ����ʯ���ƣ���Ӳ�ȱȽ��ʯ���ԭ����C-N������C-C���̣��ۼ�ǿ
C-N������C-C���̣��ۼ�ǿ
��9��Ԫ��X��Z��B��C���γɵ�Z��XBC��3������������ԭ������λ������Ҫ����1��1�ĸ������������������Ѫ��ɫ����������������ӻ�ѧʽΪ
[Fe��SCN��]2+
[Fe��SCN��]2+
����Ԫ�ط��Ŵ�����ĸ�ش𣩣������������Ļ�ѧ����ABD
ABD
A�����Ӽ� B�����Լ� C���Ǽ��Լ� D����λ����
��������Ԫ�������ڱ��е�λ�ÿ�֪��AΪH��BΪC��CΪN��DΪO��EΪNa��XΪS��YΪCl��ZΪFe��WΪCu��
��1��Y-�ĺ��������Ϊ18��ԭ����3�����Ӳ㣬����������Ϊ7��
��2��N��������Ϊ7��
��3��BΪC��ռ��1s��2s��2p�е����������
��4���ǽ�����Խǿ���縺��Խ��
��5��SO42-���ӵ�����ԭ�ӳɼ���Ϊ4���¶Ե���Ϊ
=0���ӻ�����Ϊsp3��Ϊ�������壻
��6��Cu��ԭ������Ϊ29��λ�ڵ������ڵڢ�B�壻
��7��NaH��ˮ��Ӧ����NaOH��������
��8��C3N4�����У�NΪ-3�ۣ�CΪ+4�ۣ�Ϊԭ�Ӿ��壻2��
��9��Fe��SCN��3����������ԭ������λ������Ҫ����1��1�ĸ������������������Ѫ��ɫ������Ϊ[Fe��SCN��]2+��������к����Ӽ�����λ���ͼ��Թ��ۼ���
��1��Y-�ĺ��������Ϊ18��ԭ����3�����Ӳ㣬����������Ϊ7��
��2��N��������Ϊ7��
��3��BΪC��ռ��1s��2s��2p�е����������
��4���ǽ�����Խǿ���縺��Խ��
��5��SO42-���ӵ�����ԭ�ӳɼ���Ϊ4���¶Ե���Ϊ
| 6+2-4��2 |
| 2 |
��6��Cu��ԭ������Ϊ29��λ�ڵ������ڵڢ�B�壻
��7��NaH��ˮ��Ӧ����NaOH��������
��8��C3N4�����У�NΪ-3�ۣ�CΪ+4�ۣ�Ϊԭ�Ӿ��壻2��
��9��Fe��SCN��3����������ԭ������λ������Ҫ����1��1�ĸ������������������Ѫ��ɫ������Ϊ[Fe��SCN��]2+��������к����Ӽ�����λ���ͼ��Թ��ۼ���
����⣺��Ԫ�������ڱ��е�λ�ÿ�֪��AΪH��BΪC��CΪN��DΪO��EΪNa��XΪS��YΪCl��ZΪFe��WΪCu��
��1��Y-�ĺ��������Ϊ18�������Ų�ʽΪ1s22s22p63s23p6��ԭ����3�����Ӳ㣬����������Ϊ7��λ�ڵ������ڵڢ���A�壬
�ʴ�Ϊ��1s22s22p63s23p6����������A��
��2��N��������Ϊ7����̬ԭ�Ӻ�������Ų�ʽΪ1s22s22p3���ʴ�Ϊ��1s22s22p3��
��3��BΪC��ռ��1s��2s��2p�е������������4��������ʴ�Ϊ��4��
��4���ǽ�����Խǿ���縺��Խ�縺���ɴ�С��˳����O��N��C���ʴ�Ϊ��O��N��C��
��5��SO42-���ӵ�����ԭ�ӳɼ���Ϊ4���¶Ե���Ϊ
=0���ӻ�����Ϊsp3��Ϊ�������壬�ʴ�Ϊ��sp3���������壻
��6��Cu��ԭ������Ϊ29��ԭ�Ӽ۵����Ų�Ϊ3d104s1��λ�ڵ������ڵڢ�B�壬�ʴ�Ϊ��3d104s1���ģ���B��
��7��NaH��ˮ��Ӧ����NaOH���������÷�ӦΪNaH+H2O�TNaOH+H2�����ʴ�Ϊ��NaH+H2O�TNaOH+H2����
��8��C3N4�����У�NΪ-3�ۣ�CΪ+4�ۣ�Ϊԭ�Ӿ��壬C-N������C-C���̣��ۼ�ǿ��Ӳ�ȴʴ�Ϊ��+4��C-N������C-C���̣��ۼ�ǿ��
��9��Fe��SCN��3����������ԭ������λ������Ҫ����1��1�ĸ������������������Ѫ��ɫ������Ϊ[Fe��SCN��]2+��������к����Ӽ�����λ���Ͳ�ͬ�ǽ���Ԫ��֮���γɼ��Թ��ۼ����ʴ�Ϊ��[Fe��SCN��]2+��ABD��
��1��Y-�ĺ��������Ϊ18�������Ų�ʽΪ1s22s22p63s23p6��ԭ����3�����Ӳ㣬����������Ϊ7��λ�ڵ������ڵڢ���A�壬
�ʴ�Ϊ��1s22s22p63s23p6����������A��
��2��N��������Ϊ7����̬ԭ�Ӻ�������Ų�ʽΪ1s22s22p3���ʴ�Ϊ��1s22s22p3��
��3��BΪC��ռ��1s��2s��2p�е������������4��������ʴ�Ϊ��4��
��4���ǽ�����Խǿ���縺��Խ�縺���ɴ�С��˳����O��N��C���ʴ�Ϊ��O��N��C��
��5��SO42-���ӵ�����ԭ�ӳɼ���Ϊ4���¶Ե���Ϊ
| 6+2-4��2 |
| 2 |
��6��Cu��ԭ������Ϊ29��ԭ�Ӽ۵����Ų�Ϊ3d104s1��λ�ڵ������ڵڢ�B�壬�ʴ�Ϊ��3d104s1���ģ���B��
��7��NaH��ˮ��Ӧ����NaOH���������÷�ӦΪNaH+H2O�TNaOH+H2�����ʴ�Ϊ��NaH+H2O�TNaOH+H2����
��8��C3N4�����У�NΪ-3�ۣ�CΪ+4�ۣ�Ϊԭ�Ӿ��壬C-N������C-C���̣��ۼ�ǿ��Ӳ�ȴʴ�Ϊ��+4��C-N������C-C���̣��ۼ�ǿ��
��9��Fe��SCN��3����������ԭ������λ������Ҫ����1��1�ĸ������������������Ѫ��ɫ������Ϊ[Fe��SCN��]2+��������к����Ӽ�����λ���Ͳ�ͬ�ǽ���Ԫ��֮���γɼ��Թ��ۼ����ʴ�Ϊ��[Fe��SCN��]2+��ABD��
���������⿼��λ�á��ṹ�����ʵĹ�ϵ��Ӧ�ã�Ԫ�ص��ƶ��ǽ��Ĺؼ�������ԭ�ӽṹ�����ʵĿ��飬�漰֪ʶ��϶࣬����ѡ��3��֪ʶ���ɣ���Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ








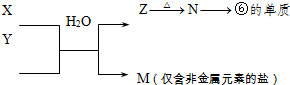
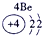
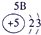
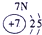
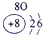
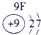
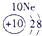
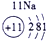





 ��ʾ����
��ʾ����