��Ŀ����
ij��ɫ��Һ�к���K+��Cl-��OH-��SO32����SO42����Ϊ������Һ�������ĸ��������ӣ����õ��Լ��У����ᡢ���ᡢ��������Һ�����ᱵ��Һ����ˮ�ͷ�̪��Һ����������OH-��ʵ�鷽�����ԣ��������������ӵĹ�������ͼ��ʾ��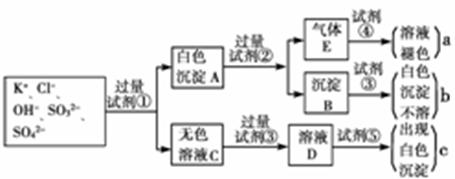
(1)ͼ���Լ��١������ʵĻ�ѧʽ�ֱ��ǣ�
��_________����__________����____________����_________����_________________��
(2)ͼ������a��b��c��������������ӷֱ��ǣ�A____________��b____________��c_______________��
(3)��ɫ����A���Լ��ڷ�Ӧ�����ӷ���ʽ��_________________________��
(4)��ɫ��ҺC���Լ��۵���ҪĿ����___________________________��
(5)��ɫ����A�����Լ��۶������Լ��ڣ���ʵ���Ӱ����__________________________��
(6)����Eͨ���Լ��ܷ�����Ӧ�����ӷ���ʽ��____________________________________��
(1)Ba(NO3)2 HCl HNO3 Br2 AgNO3 (2) SO32����SO42����Cl-
(3)BaSO3+2H+��Ba2++SO2��+H2O (4)�к�OH-����ֹ��Cl-�ļ����������
(5)��ʹSO32����SO42���ļ���������ţ�����ȷ��SO32����SO42���Ƿ���� (6)SO2+Br2+2H2O��4H++ SO42��+2Br-
����������������ݰ�ɫ����A���Լ��ڷ�Ӧ������E���ɿ�֪��A�к����������Σ�EӦ����SO2�������Լ��������ᱵ����A�к������ᱵ�������ᱵ���Լ��������ᣬ���������ᣬ��Ϊ�������ǿ�����ԡ�SO2��ʹ�Լ�����ɫ��˵���Լ���Ӧ������ˮ��B�����ᱵ�������������У����Լ��������ᡣC�к��������ӡ��������Լ�OH-����OH-�ܸ��������ӵļ��飬����Ҫ�ȼ��������кͼȻ���ټ�����������Һ���������ӣ����Լ�������������
���㣺�������ӹ����Լ����ӵļ���
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣�����ۺ���ǿ���������У����ض�ѧ�������������ͷ�����ָ��������������ѧ���淶���Ͻ���ʵ��������������ѧ�����ۺ�ʵ������������ѧ����ѧ�����������������ص㿼��ʵ����������Ĺ淶�Ժ�ȷ�Լ��������֪ʶ���ʵ�������������

 ���ѵ����Ԫ��ĩ���100��ϵ�д�
���ѵ����Ԫ��ĩ���100��ϵ�д� ��˼άС�ھ�100����ҵ��ϵ�д�
��˼άС�ھ�100����ҵ��ϵ�д� ��ʦָ��һ��ͨϵ�д�
��ʦָ��һ��ͨϵ�д�| O | 2- 3 |
| O | 2- 4 |

�����йؽ��۴�����ǣ�������
| A���Լ�����AgNO3��Һ���Լ�����HNO3������1�а�ɫ������AgCl | ||
| B������3�а�ɫ������BaSO4 | ||
C����������2�����ӷ���ʽ�ǣ�Br2+2H2O+SO2=4H++2Br-+S
| ||
| D���Լ��������ᣬ�Լ��������� |



 ��
�� ��Ϊ����ȷ�����������ĸ��������ӣ����õ��Լ��У�ϡ���ᡢϡ���ᡢ��������Һ�����ᱵ��Һ������ʯ��ˮ�ͷ�̪��Һ�����м���OH����ʵ�鷽�����ԣ���֪�������������ӵĹ�������ͼ��ʾ��
��Ϊ����ȷ�����������ĸ��������ӣ����õ��Լ��У�ϡ���ᡢϡ���ᡢ��������Һ�����ᱵ��Һ������ʯ��ˮ�ͷ�̪��Һ�����м���OH����ʵ�鷽�����ԣ���֪�������������ӵĹ�������ͼ��ʾ��