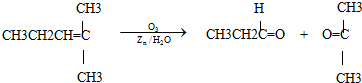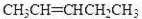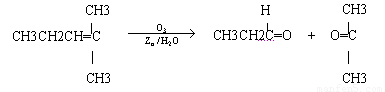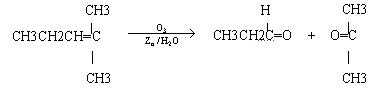��Ŀ����
ϩ��ͨ��������������п��ˮ�����õ�ȩ��ͪ�����磺
��. ��֪��ȩ��ȼ����Ϊ1 815 kJ��mol-1����ͪ��ȼ����Ϊ1 789 kJ��mol-1����д����ȩȼ�յ��Ȼ�ѧ����ʽ______________________________��?
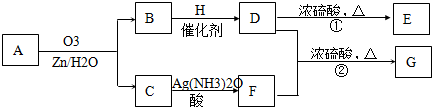
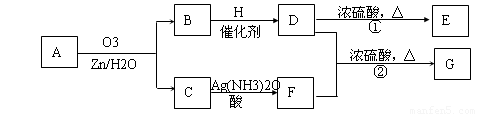
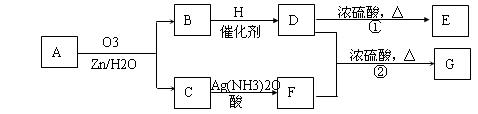
��. ������Ӧ�������ƶ�ϩ���Ľṹ��һ����״��ϩ��Aͨ��������������п��ˮ�����õ�B��C��������B��̼69.8%������11.6%��B��������Ӧ������������D��D��Ũ��������¼��ȣ��ɵõ���ʹ��ˮ��ɫ��ֻ��һ�ֽṹ������E����Ӧͼʾ���£�
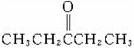
����������⣺?
��1��B����Է���������__________��C��F�ķ�Ӧ����Ϊ__________����D�к��й����ŵ�����__________��?
��2��D+F��G�Ļ�ѧ����ʽ�ǣ�______________________________________________��
��3��A�Ľṹ��ʽΪ________________________________��?
��4��������A��ij��ͬ���칹��ͨ��������������п��ˮ����ֻ�õ�һ�ֲ�����ϸ��������칹��Ľṹ��ʽ��__________�֡�
��������.ȼ���ȶ���Ϊ1 mol����ȼ�������ȶ�������ʱ�ų������������ݱ�ȩ��ȼ����Ϊ1 815 kJ��mol-1��д���Ȼ�ѧ����ʽ��CH3CH2CHO(l)+4O2(g)![]() 3CO2(g)+3H2O(l)����H=-1 815 kJ��mol-1��
3CO2(g)+3H2O(l)����H=-1 815 kJ��mol-1��
��.(1)1������״��ϩ��A���������̣��õ�2���ӱ���һԪȩ(��ͪ)�����Կ��Լ���B�ķ�����ɵ�CnH2nO������![]() ��100%=69.8%���õ�n=5��B����Է�������Ϊ86��C��F�ı仯ʹ����������Һ������CΪȩ��������������Ӧ��������FΪ���ᡣB���ܷ���������Ӧ����BΪͪ����H2��Ӧ���ɴ�D����D���еĹ�����Ϊ�ǻ������Է�����ȥ��Ӧ����ϩ��E��Ҳ���Ժ�����F����������Ӧ��������G��
��100%=69.8%���õ�n=5��B����Է�������Ϊ86��C��F�ı仯ʹ����������Һ������CΪȩ��������������Ӧ��������FΪ���ᡣB���ܷ���������Ӧ����BΪͪ����H2��Ӧ���ɴ�D����D���еĹ�����Ϊ�ǻ������Է�����ȥ��Ӧ����ϩ��E��Ҳ���Ժ�����F����������Ӧ��������G��
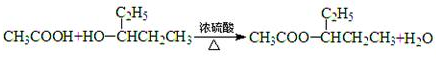
(2)��G��8��Cԭ�ӣ���D����5��Cԭ�ӣ���������F����3��Cԭ�ӣ�F�DZ��ᣬ�ṹʽΪCH3CH2COOH����D��ȥֻ�ܵõ�һ��ϩ����DΪ3-�촼���ṹΪ(C2H5)2CHOH��D��F��Ӧ��
CH3CH2COOH+(C2H5)2CHOH![]() CH3CH2COOCH(C2H5)2+H2O��
CH3CH2COOCH(C2H5)2+H2O��
(3)A������õ�B( ![]() )��C(CH3CH2CHO)����֪A�ĽṹΪ��?(CH3CH2)
)��C(CH3CH2CHO)����֪A�ĽṹΪ��?(CH3CH2)
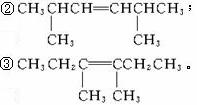
(4)���ܵĽṹ�У�
��CH3CH2CH2CH=CHCH2CH2CH3��
�𰸣���.CH3CH2CHO��l��+4O2��g��![]() 3CO2��g��+3H2O��l��?��H=-1 815 kJ��mol-1
3CO2��g��+3H2O��l��?��H=-1 815 kJ��mol-1
��.��1��86��������Ӧ���ǻ�
��2��CH3CH2COOH+��C2H5��2CHOH![]() CH3CH2COOCH��C2H5��2+H2O
CH3CH2COOCH��C2H5��2+H2O
��3����CH3CH2��
��4��3

 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�