��Ŀ����
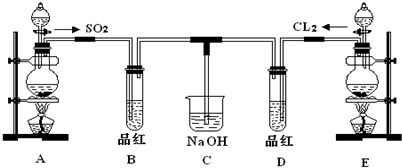
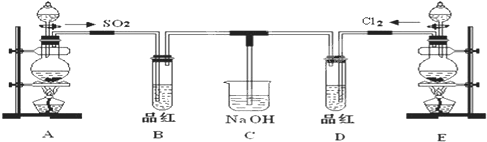
��16�֣�ij��ѧʵ��С���ͬѧΪ̽���ͱȽ�SO2����ˮ��Ư���ԣ���������µ�ʵ��װ�ã�ͼ3����
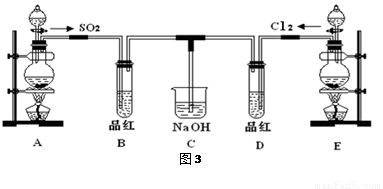
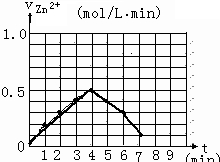
��1���ٷ�Ӧ��ʼһ��ʱ��۲쵽B��D�����Թ��е�Ʒ����Һ���ֵ������ǣ�
B��________________________________��D��____________________________��
��ֹͣͨ����,�ٸ�B��D�����Թֱܷ���ȣ������Թ��е�����ֱ�Ϊ
B��________________________________��D��____________________________��
��2����һ��ʵ��С���ͬѧ��ΪSO2����ˮ����Ư���ԣ�����Ϻ��Ư���Կ϶����ǿ�����ǽ��Ƶõ�SO2��Cl2��1:1ͬʱͨ�뵽Ʒ����Һ�У����������ɫЧ��������������������������������ԭ���û�ѧ����ʽ��ʾ��______________________________ ��
��3��װ��E����MnO2��Ũ���ᷴӦ�Ƶ�Cl2������Ӧ���ɵ�Cl2���Ϊ2.24L����״��������������HClΪ mol��
��4��ʵ���������ͬѧ��Ϊװ��C�п��ܺ���SO32����SO42����Cl����OH���������ӣ�����д��������SO42����SO32����ʵ�鱨�档
��ѡ�Լ���2 mol��L��1 HCl��1 mol��L��1 H2SO4��l mol��L��1 BaCl2��l mol��L��1MgCl2
1 mol��L��1 HNO3��0.1 mol��L��1 AgNO3�����Ʊ�����ˮ��
|
��� |
ʵ����� |
Ԥ������ͽ��� |
|
����� |
ȡ��������Һ���Թ��У����� ������
|
��֤������Һ�к�SO32���� |
|
����� |
�ڲ���ٵ���Һ�е�������
|
�� ֤������Һ�к�SO42���� |
���ڣ�4��С��ÿ��1�֣�����ÿ��2�֣���1����B��Ʒ����Һ��ɫ��D��Ʒ����Һ��ɫ��
��B����ɫ��Ʒ���ָֻ�Ϊ��ɫ��D������������
��2��Cl2��SO2��2H2O=2HCl��H2SO4 ��3��0.2
��4��
|
��� |
ʵ����� |
Ԥ������ͽ��� |
|
����� |
2 mol��L-1HCl |
�����ݲ��� |
|
����� |
l mol��L-1BaCl2 |
�а�ɫ�������� |
����������1����SO2����Ư���ԣ���ʹƷ����Һ��ɫ����ˮ����ǿ�����ԣ�Ҳ��ʹƷ����Һ��ɫ��
������SO2��Ư�����ǿ���ģ����Լ�����ʹƷ����Һ�ָ���ɫ������ˮ��Ư�������������µģ��Dz�����ģ����Ȳ��ָܻ���ɫ��
��2������SO2�����л�ԭ�ԣ����������������ԣ����߷���������ԭ��Ӧ�����Ȼ�������ᣬ����ʽΪCl2��SO2��2H2O=2HCl��H2SO4��
��3��2.24L�����ڱ�״���µ����ʵ�����0.1mol��������ԭ���غ��֪�����������Ȼ�����0.2mol��
��4��SO32���ļ���������ᣬ��Ӧ������SO2���塣SO42���ļ������Ȼ�����Һ�����ɰ�ɫ�������ᱵ��

 ��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�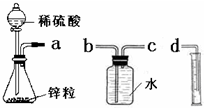 ij��ѧʵ��С���ͬѧ������������װ������ȫ��ͬ��װ�ö���̽��Ũ�ȶԷ�Ӧ���ʵ�Ӱ�죮
ij��ѧʵ��С���ͬѧ������������װ������ȫ��ͬ��װ�ö���̽��Ũ�ȶԷ�Ӧ���ʵ�Ӱ�죮��1��Ϊ�ﵽ��ʵ��Ŀ����װ������˳��Ϊ��
a��
��2�����Ӻ�װ�ú����һ��������
��3����ƿ�з�����Ӧ�����ӷ���ʽΪ
��4������װ�õķ�Һ©����װ���Լ��ֱ�Ϊ1mol/L�����4mol/L���ᣬ��С��ͬѧҪ�ⶨ����¼���������±���
| ������Լ� | H2���������ͬ�����£� | ��Ӧʱ�� | ��Ӧ���� |
| 1mol/L���� | 10mL | t1 | v1 |
| 4mol/L���� | 10mL | t2 | v2 |
��5������һ��ͬѧ�ⶨ��ÿ��һ���ӣ���ƿ�������Ũ�ȣ���¼������£�
| ʱ�䣨min�� | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| ����Ũ�ȣ�mol/L | 4.0 | 3.8 | 3.5 | 3.1 | 2.6 | 2.2 | 1.9 | 1.8 | �� |
�����0��4mimʱ�û�ѧ��Ӧ������ʱ��仯��ԭ��
��6��������ʵ�鷽���ɶ����ⶨ�÷�Ӧ�Ļ�ѧ��Ӧ�����⣬�������е�ʵ��ⶨ�����У�