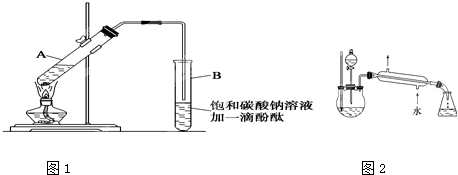
��Ŀ����
(6��)(1)ijѧ�������к��Ȳⶨ��ȡ��50 mL 0��50 mol��L��1��HCl��50 mL 0��55 mol��L��1 ��NaOH��Һ(�ܶȶ���1 g��cm��3)��ʵ���õ��������ݣ�

(�кͺ����ɵ���Һ�ı�����Ϊ4��18J��g��1������1)�����ͬѧ������к��ȵ�ƽ��ֵ����������������������������������������������������������
(2)��ѧ����õ����ݱ�����ֵ��������(��ߡ��͡�)��
(3)�����з�����ѡ����ѧ������ʵ������ԭ�������(��д��ĸ)����������
A����Һ��Ϻ�δ��ʱ�Ǻ����ȼƱ���
B���㵹��Һ̫�죬�����������ձ�
C����Һ��Ϻ���費��
D��δ���¶��������ֵ�ͼ�¼�¶ȼ�ʾ��
E������Ͳ��ȡ�������ʱ���Ӷ���
F���ձ��Ͳ�����������һ��������
(1)52.25kJ��mol��1 (2)�� (3)ABCDF
����������1������ʵ�����ݿ�֪��ʵ��3����������Ч�ģ������¶Ȳ��ƽ��ֵ��3.125�棬���Ը��ݡ�H��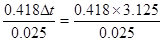 ��52.25kJ��mol��1��
��52.25kJ��mol��1��
��2���к�����57.3kJ��mol��1������ʵ��ֵ������ֵ�͡�
��3����ֵƫ�ͣ�˵����Ӧ����������ʧ���������ᡢ������ʧ��E�ж���ƫ���Ӧ������ƫ�ߣ�������ȷ�Ĵ�ѡABCDF��

�����6�֣�
ijѧ��������ȤС��Ϊ�˲ⶨþ���Ͻ������ĺ���������������ʵ�顣���Ͻ�3.0��Ͷ�뵽������100 mL 1.5 mol?L-1���ռ���Һ�У���ַ�Ӧ������δ��Ӧ��þ��Ȼ������Һ�еμ�1.0 mol?L-1�����ᣬ��������������õ��������������±���
| ʵ����� | ������������ | �������� |
| 1 | 60 mL | 0 |
| 2 | 80 mL | 0.78 g |
| 3 | 180 mL | 5.46 g |
��2���Ͻ���������������Ϊ ��
(6��)(1)ijѧ�������к��Ȳⶨ��ȡ��50 mL 0��50 mol��L��1��HCl��50 mL 0��55 mol��L��1��NaOH��Һ(�ܶȶ���1 g��cm��3)��ʵ���õ��������ݣ�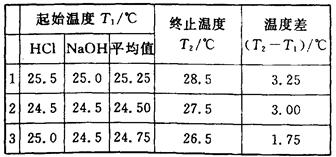
(�кͺ����ɵ���Һ�ı�����Ϊ4��18J��g��1������1)�����ͬѧ������к��ȵ�ƽ��ֵ����������������������������������������������������������
(2)��ѧ����õ����ݱ�����ֵ��������(��ߡ��͡�)��
(3)�����з�����ѡ����ѧ������ʵ������ԭ�������(��д��ĸ)����������
| A����Һ��Ϻ�δ��ʱ�Ǻ����ȼƱ��� |
| B���㵹��Һ̫�죬�����������ձ� |
| C����Һ��Ϻ���費�� |
| D��δ���¶��������ֵ�ͼ�¼�¶ȼ�ʾ�� |
F���ձ��Ͳ�����������һ��������
�����6�֣�
ijѧ��������ȤС��Ϊ�˲ⶨþ���Ͻ������ĺ���������������ʵ�顣���Ͻ�3.0��Ͷ�뵽������100 mL 1.5 mol•L-1���ռ���Һ�У���ַ�Ӧ������δ��Ӧ��þ��Ȼ������Һ�еμ�1.0 mol•L-1�����ᣬ��������������õ��������������±���
|
ʵ����� |
������������ |
�������� |
|
1 |
60 mL |
0 |
|
2 |
80 mL |
0.78 g |
|
3 |
180 mL |
5.46 g |
��1����ʼ����ʱ������������������� mL�����õ������������ʱ��������������Ϊ mL��
��2���Ͻ���������������Ϊ ��