��Ŀ����
��һ�����Һ��ֻ���ܺ������������е������֣�K+��NH4+��Cl-��Mg2+��Ba2+��CO32-��SO42-����ȡ����100mL��Һ��������ʵ�飺
��1�����һ����Һ�м���AgNO3��Һʱ�г���������
��2����ڶ�����Һ�м�������NaOH��Һ�����Ⱥ��ռ�������0.06mol��
��3�����������Һ�м�������BaCl2��Һ�����ó�����ϴ�Ӹ������Ϊ6.27g������������ϴ�ӡ������������Ϊ2.33g����������ʵ�飬�����Ʋ���ȷ���ǣ�������
��1�����һ����Һ�м���AgNO3��Һʱ�г���������
��2����ڶ�����Һ�м�������NaOH��Һ�����Ⱥ��ռ�������0.06mol��
��3�����������Һ�м�������BaCl2��Һ�����ó�����ϴ�Ӹ������Ϊ6.27g������������ϴ�ӡ������������Ϊ2.33g����������ʵ�飬�����Ʋ���ȷ���ǣ�������
| A��K+һ������ |
| B��100ml��Һ�к�0.01molCO32- |
| C��Cl-���ܴ��� |
| D��Ba2+һ�������ڣ�Mg2+���ܴ��� |
��һ�ݼ���AgNO3��Һ�г������������ܷ���Cl-+Ag+�TAgCl����CO32-+2Ag+�TAg2CO3����SO42-+2Ag+�TAg2SO4�������Կ��ܺ���Cl-��CO32-��SO42-��
�ڶ��ݼ�����NaOH��Һ���Ⱥ��ռ�������0.06mol���ܺ�NaOH��Һ���Ȳ��������ֻ����NH4+�����ݷ�ӦNH4++OH-
NH3��+H2O������NH3Ϊ0.06mol���ɵ�NH4+ҲΪ0.06mol��
�����ݼ�����BaCl2��Һ�ø������6.27g������������ϴ�ӣ������������Ϊ2.33g�����ֳ����������ᣬΪBaCO3�����ֳ������������ᣬΪBaSO4��������ӦCO32-+Ba2+�TBaCO3����SO42-+Ba2+�TBaSO4���������Һ��һ������CO32-��SO42-��һ��������Ba2+��Mg2+��
��������֪BaSO4Ϊ2.33g�����ʵ���Ϊ
=0.01mol����SO42-�����ʵ���Ϊ0.01mol��BaCO3Ϊ6.27g-2.33g�T3.94g�����ʵ���Ϊ
=0.02mol����CO32-���ʵ���Ϊ0.02mol��
�����������ɵã���Һ��һ������CO32-��SO42-��NH4+��һ��������Mg2+��Ba2+����CO32-��SO42-��NH4+���ʵ����ֱ�Ϊ0.02mol��0.01mol��0.06mol��
A��CO32-��SO42-���������Ϊ0.02mol��2+0.01mol��2=0.06mol��NH4+���������Ϊ0.06mol��������Һ�е���غ㣬��֪K+��һ�����ڣ���A����
B�������������ɵã�100mL��Һ��CO32-���ʵ���Ϊ0.02mol����B����
C��CO32-��SO42-���������Ϊ0.02mol��2+0.01mol��2=0.06mol��NH4+���������Ϊ0.06mol��������Һ�е���غ㣬��֪Cl-��K+���ܰ�1��1���ڣ���C��ȷ��
D��������������֪����Һ��һ������CO32-��SO42-��NH4+��һ��������Mg2+��Ba2+����D����
��ѡC��
�ڶ��ݼ�����NaOH��Һ���Ⱥ��ռ�������0.06mol���ܺ�NaOH��Һ���Ȳ��������ֻ����NH4+�����ݷ�ӦNH4++OH-
| ||
�����ݼ�����BaCl2��Һ�ø������6.27g������������ϴ�ӣ������������Ϊ2.33g�����ֳ����������ᣬΪBaCO3�����ֳ������������ᣬΪBaSO4��������ӦCO32-+Ba2+�TBaCO3����SO42-+Ba2+�TBaSO4���������Һ��һ������CO32-��SO42-��һ��������Ba2+��Mg2+��
��������֪BaSO4Ϊ2.33g�����ʵ���Ϊ
| 2.33g |
| 233g/mol |
| 3.94g |
| 197g/mol |
�����������ɵã���Һ��һ������CO32-��SO42-��NH4+��һ��������Mg2+��Ba2+����CO32-��SO42-��NH4+���ʵ����ֱ�Ϊ0.02mol��0.01mol��0.06mol��
A��CO32-��SO42-���������Ϊ0.02mol��2+0.01mol��2=0.06mol��NH4+���������Ϊ0.06mol��������Һ�е���غ㣬��֪K+��һ�����ڣ���A����
B�������������ɵã�100mL��Һ��CO32-���ʵ���Ϊ0.02mol����B����
C��CO32-��SO42-���������Ϊ0.02mol��2+0.01mol��2=0.06mol��NH4+���������Ϊ0.06mol��������Һ�е���غ㣬��֪Cl-��K+���ܰ�1��1���ڣ���C��ȷ��
D��������������֪����Һ��һ������CO32-��SO42-��NH4+��һ��������Mg2+��Ba2+����D����
��ѡC��

��ϰ��ϵ�д�
 ȫ�ܲ��һ���þ�ϵ�д�
ȫ�ܲ��һ���þ�ϵ�д�
�����Ŀ
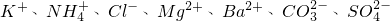 ����ȡ����100mL��Һ��������ʵ�飺
����ȡ����100mL��Һ��������ʵ�飺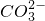
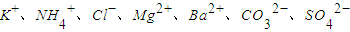 ����ȡ����100mL��Һ��������ʵ�飺
����ȡ����100mL��Һ��������ʵ�飺