��Ŀ����
����Ŀ����84����Һ����������Һ��Ӧ������ȡ��������Ӧ����ʽΪNaClO+NaCl+H2SO4![]() Na2SO4+Cl2��+
Na2SO4+Cl2��+
H2O��Ϊ̽�����������ʣ�ijͬѧ�����������ʾ��ʵ��װ�á�
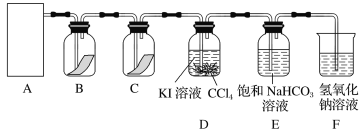
��ش�
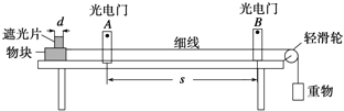
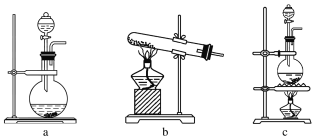
(1)�ڸ�ʵ���У���ȡ������װ����________(����ĸ)��
(2)װ��B��C�����ηŵ��Ǹ���ĺ�ɫ������ʪ��ĺ�ɫ������ʵ������и�ͬѧ����װ��B�еIJ���Ҳ��ɫ����ԭ�������________________________________________________������������ĸĽ�����_______________________________________________��
(3)D�е�������_____________________����Ӧ�����ӷ���ʽΪ_________________________________��
����D��Һ��ķ�����___________________________________________��
(4)д��������NaOH��Һ��Ӧ�����ӷ���ʽ______________________________���÷�Ӧ���������뻹ԭ�������ʵ���֮��Ϊ________��Ϊ��֤β�����պ����Һ�д���Cl����ȷ�IJ�����____________________��
(5)����ͨ�뱥��NaHCO3��Һ�ܲ�����ɫ���壬��֪���ԣ����̼������ᣬ��ʵ��֤��Cl2��H2O��Ӧ�IJ����к���________��
���𰸡�(1)c
(2)Cl2�л�������H2O(g) ��A��B֮������װ��ŨH2SO4��ϴ��ƿ
(3)�²�CCl4����Ϻ�ɫ Cl2+2I===I2+2Cl ��Һ
(4)Cl2+2OH===Cl+ClO+H2O 1��1 ȡ�����ձ�������Cl2�����Һ������������ϡHNO3�ữ���ټ���AgNO3��Һ�����а�ɫ�������ɣ�֤�����к���Cl
(5)����
��������(1)���ݡ���+Һ![]() ����ԭ����֪Ӧѡ��cװ����ȡCl2��
����ԭ����֪Ӧѡ��cװ����ȡCl2��
(2)������ɫ֤��Cl2�л���H2O(g)��������HClO��Ӧ���ӳ�H2O(g)��װ�á�
(3)Cl2+2I===I2+2Cl��CCl4��I2��ȡ������I2����CCl4����Һ���Ϻ�ɫ���������ֻ������ܵ�Һ����÷�Һ����
(4)Cl2��NaOH��Һ��Ӧ�����ӷ���ʽΪCl2+2OH===Cl+ClO+H2O�����ݷ�Ӧǰ����Ԫ�ػ��ϼ۱仯��֪Cl2������������������ԭ���������ʵ���֮��Ϊ1��1������Cl��Ӧ����ϡHNO3�ữ�к���Һ�е�OH������ClOת��ΪHClO���ټ�AgNO3��Һ������AgCl��ɫ�������ɣ�֤������Cl��
(5)������HCl��H2CO3��HClO��HCl��NaHCO3��Ӧ��HCl+NaHCO3===NaCl+H2O+CO2������HClO����NaHCO3��Ӧ����������H2O��Ӧ�IJ����к����ᡣ

 ���Ͱ�ͨ��ĩ���ϵ�д�
���Ͱ�ͨ��ĩ���ϵ�д�