ĢāÄæÄŚČŻ
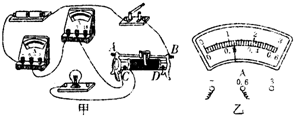
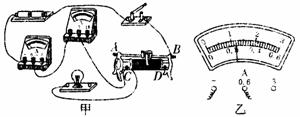
ČēĶ¼ĖłŹ¾ŹĒŠ”Ī°×é²āĮæ±źÓŠ”°2.5V”±µÄŠ”µĘÅŻµē×čµÄŹµĪļĮ¬ĻßĶ¼£®£ØŅŃÖŖ»¬¶Æ±ä×čĘ÷×ī“ó×čÖµĪŖR0£©
£Ø1£©ĒėÓĆĒ¦±Ź»Ļß“śĢęµ¼Ļß½«µēĀ·²¹³äĶźÕū£®
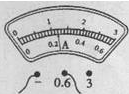
£Ø2£©Š”Ī°¼ģ²éµēĀ·Į¬½ÓĪŽĪóŗ󣬱ÕŗĻæŖ¹Ų£¬·¢ĻÖŠ”µĘÅŻ²»ĮĮ£¬Į¢¼“¾ŁŹÖŅŖĒóĄĻŹ¦øü»»µĘÅŻ£¬ÕāÖÖ×ö·Ø________£ØŃ”Ģī”°Ķ×µ±”±»ņ”°²»Ķ×µ±”±£©£®¾Ķ¬×é³ÉŌ±ĢįŹ¾»¹·¢ĻÖµēŃ¹±ķĪŽŹ¾Źż”¢µēĮ÷±ķÓŠŹ¾Źż£¬ŌņµĘÅŻ²»ĮĮµÄŌŅņæÉÄÜŹĒ_____________________________£®ĪŹĢā½ā¾öŗ󣬊”Ī°µ÷½Ś»¬¶Æ±ä×čĘ÷£¬Ź¹Š”µĘÅŻÕż³£¹¤×÷£¬µēĮ÷±ķŹ¾ŹżČēĶ¼ĖłŹ¾£¬ŌņŠ”µĘÅŻ“ĖŹ±µÄµē×čŌ¼ĪŖ________¦ø£®
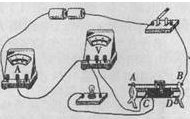
£Ø3£©Ķź³ÉŹµŃéŗ󣬊”Ī°ĻėÓĆŌµēĀ·²āijŅ»¶ØÖµµē×čRxµÄ×čÖµ£¬ĖūÓĆøƵē×čĢę»»µĘÅŻŗ󣬷¢ĻÖµēŃ¹±ķĖš»µĮĖ£¬±ć½«Ę䲚³ż£®ĒėÄćŌŚ²»øıäĘäĖūŌŖ¼žĮ¬½ÓµÄ·½Ź½ĻĀ£¬°ļĖūĶź³ÉŹµŃé²½Öč£ŗ
A£®________________________![]() ________________£»
________________£»
B£®________________________________________£®
±ķ“ļŹ½£ŗRx=________________£®£ØÓĆĖł²āĪļĄķĮæ·ūŗűķŹ¾£©£®
£Ø1£©ĀŌ £Ø2£©²»Ķ×µ±£»µĘÅŻ¶ĢĀ·£»10.4 £Ø3£©½«»¬Ę¬Ņʵ½×ī×ó¶Ė£¬¶Į³öµēĮ÷±ķµÄŹ¾ŹżĪŖI1£»½«»¬Ę¬Ņʵ½×īÓŅ¶Ė£¬¶Į³öµēĮ÷±ķµÄŹ¾ŹżĪŖI2£»![]()

 ŗ®¼Ł““ŠĀŠĶ×ŌÖ÷ѧĻ°µŚČżŃ§ĘŚŗ®¼ŁĻĪ½ÓĻµĮŠ“š°ø
ŗ®¼Ł““ŠĀŠĶ×ŌÖ÷ѧĻ°µŚČżŃ§ĘŚŗ®¼ŁĻĪ½ÓĻµĮŠ“š°ø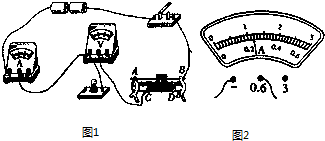 £Ø2013?ɽĪ÷£©ČēĶ¼1ŹĒŠ”Ī°×é²āĮæ±źÓŠ”°2.5V”±µÄŠ”µĘÅŻµē×čµÄŹµĪļĮ¬ĻßĶ¼£®£ØŅŃÖŖ»¬¶Æ±ä×čĘ÷×ī“ó×čÖµĪŖR0£©
£Ø2013?ɽĪ÷£©ČēĶ¼1ŹĒŠ”Ī°×é²āĮæ±źÓŠ”°2.5V”±µÄŠ”µĘÅŻµē×čµÄŹµĪļĮ¬ĻßĶ¼£®£ØŅŃÖŖ»¬¶Æ±ä×čĘ÷×ī“ó×čÖµĪŖR0£©