��Ŀ����
40��ij������Ʒ�к��������Ȼ�þ���Ȼ��ƣ�С������������ᴿ������
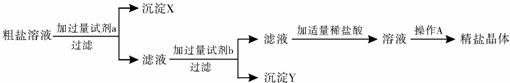
��1�����Լ�aΪ�������ƣ��Լ�b�Ļ�ѧʽΪ
��2��ָ������A������
��3�������м�����ϡ����ʱ��������Ӧ�Ļ�ѧ����ʽΪ
��4������ͼ��ʾװ�ý��й���ʱ�������������עҺ��ʱ��������Ӧ
A��������������ģ�B���¶˿�ס������������λ�ã�C���¶˿�ס��������������ֽһ��
��5����һ�ι��˺�����Һ�Ի��ǣ�����ֽδ��������Ž��еIJ���ʱ

��1�����Լ�aΪ�������ƣ��Լ�b�Ļ�ѧʽΪ
Na2CO3
����2��ָ������A������
����
����3�������м�����ϡ����ʱ��������Ӧ�Ļ�ѧ����ʽΪ
NaOH+HCl�TNaCl+H2O Na2CO3+2HCl�T2NaCl+H2O+CO2��
����4������ͼ��ʾװ�ý��й���ʱ�������������עҺ��ʱ��������Ӧ
C
������ţ���A��������������ģ�B���¶˿�ס������������λ�ã�C���¶˿�ס��������������ֽһ��
��5����һ�ι��˺�����Һ�Ի��ǣ�����ֽδ��������Ž��еIJ���ʱ
�ٹ���һ��
����������1�����д�����Һ�ӹ����Լ��������ƹ��ˣ��ó���������þ���ӹ����Լ�̼���ƹ��˵ã���Һ��̼��ơ�̼��þ���������������ơ�̼���ƣ��������������õ�����ʳ�Σ�
��2�������ᴿ��ȷ����Ϊ�ܽ⡢���ˡ�������������ʣ��ٴ�������ʢ��ˮ���ձ��бӱ��ò��������裬ֱ���ܽ⣻�ڹ���ʱע�����һ�������ͣ�������������һ�ι��˺�����Һ�Ի��ǣ�����ֽδ��������Ž��еIJ���ʱ����ι��ˣ�
��2�������ᴿ��ȷ����Ϊ�ܽ⡢���ˡ�������������ʣ��ٴ�������ʢ��ˮ���ձ��бӱ��ò��������裬ֱ���ܽ⣻�ڹ���ʱע�����һ�������ͣ�������������һ�ι��˺�����Һ�Ի��ǣ�����ֽδ��������Ž��еIJ���ʱ����ι��ˣ�
����⣺��1����̼��������ơ�þ���ӣ���2�������ᴿ��ȷ����Ϊ���ܽ⣺��������ʢ��ˮ���ձ��бӱ��ò��������裬ֱ���ܽ⣻�ڹ��ˣ�ע�����һ�������ͣ�����������������ע���н϶�������ʱ����ֹͣ���ȣ��ܼ�����ʣ���3���������������ơ�̼���ƣ������������4������ʱע���������֮һ���������¶˿�ס��������������ֽһ�ߣ���5����һ�ι��˺�����Һ�Ի��ǣ�����ֽδ��������Ž��еIJ���ʱ����ι��ˣ�
�ʴ�Ϊ����1��Na2CO3��2������ ��3��NaOH+HCl�TNaCl+H2O Na2CO3+2HCl�T2NaCl+H2O+CO2�� ��4��C ��5���ٹ���һ��
�ʴ�Ϊ����1��Na2CO3��2������ ��3��NaOH+HCl�TNaCl+H2O Na2CO3+2HCl�T2NaCl+H2O+CO2�� ��4��C ��5���ٹ���һ��
������ע������ķ��뷽����̽��ʵ�������ע�����һ�������ͣ��������������������������ƺ�ѡ�ã���ȷ��д���ڳ����ʵĻ�ѧ����ʽ��

��ϰ��ϵ�д�
 ǧ�������������ĩ�����Ծ�����ϵ�д�
ǧ�������������ĩ�����Ծ�����ϵ�д�
�����Ŀ





