��Ŀ����
ijУ��ѧ��ȤС���ͬѧ�Գ��ڷ��õĹ����ռ�ı��ʳ̶ȿ�չ��̽����
��1��д���ռ�����ʷ�Ӧ�Ļ�ѧ����ʽ��________________________________________ Ϊȷ������������̼���Ƶ�����������������������¼��ֲ�ͬ��ʵ�鷽����
��2�� [����һ]�����������
����ͼ1��ʾ�����мгֵ�������������Ħ��ע����������X g�Ļ����������ϡ���ᷴӦ�ⶨ������CO2��������������װ�õ������Եķ�����________________��
��1��д���ռ�����ʷ�Ӧ�Ļ�ѧ����ʽ��________________________________________ Ϊȷ������������̼���Ƶ�����������������������¼��ֲ�ͬ��ʵ�鷽����
��2�� [����һ]�����������
����ͼ1��ʾ�����мгֵ�������������Ħ��ע����������X g�Ļ����������ϡ���ᷴӦ�ⶨ������CO2��������������װ�õ������Եķ�����________________��
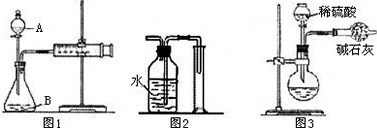
����ͬѧ�����ͼ2����ͼ1�е��ռ�װ�ã�������CO2��������________(�ƫ����ƫС���������䡱)��������______________________________________����ĸĽ�������________________________________________________________________�����ƿ��ԭ�еĿ�����ʵ����_______(��С���û�С�)Ӱ�졣
��Ҳ������ͼ3װ�òⶨCO2������ (��ʯ�ҵijɷ���CaO��NaOH�Ļ����)����ͼ3ʵ��װ����Ҫ������Щȱ��?��Щȱ�ݶ�ʵ�����к�Ӱ��?(ѡ1�������±�)
��Ҳ������ͼ3װ�òⶨCO2������ (��ʯ�ҵijɷ���CaO��NaOH�Ļ����)����ͼ3ʵ��װ����Ҫ������Щȱ��?��Щȱ�ݶ�ʵ�����к�Ӱ��?(ѡ1�������±�)

��3��[������]������������
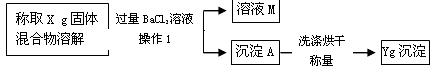
�١�����1����������_____________��
��ȷ��BaCl2��Һ�Ƿ�����ķ����� _______________________________��
�۵��²ⶨ�Ľ��ƫ���ԭ�������_______________________________________��
��ȷ��BaCl2��Һ�Ƿ�����ķ����� _______________________________��
�۵��²ⶨ�Ľ��ƫ���ԭ�������_______________________________________��
��1��2NaOH+CO2===Na2CO3+H2O
��2��[����һ]
�ٽ�ע������������һ�����룬һ��ʱ����ɿ������������ܻص�ԭλ��©��
��ƫС�� CO2������ˮ����ˮ��Ӧ�� ��ˮ���ϸ���һ��ֲ���ͣ����ƿ�е�ˮ��Ϊ���͵�CO2ˮ��Һ�Ⱥ����𰸣��� û��
�� �������𰸺������ɣ�
�������𰸺������ɣ�
��3��[������]
�ٹ���
�ھ��ã����ϲ���Һ�м����μ�BaCl2��Һ��������˵��BaCl2��Һ�ѹ���
�۳���δϴ�Ӹɾ�����������ﲻ��֣�
��2��[����һ]
�ٽ�ע������������һ�����룬һ��ʱ����ɿ������������ܻص�ԭλ��©��
��ƫС�� CO2������ˮ����ˮ��Ӧ�� ��ˮ���ϸ���һ��ֲ���ͣ����ƿ�е�ˮ��Ϊ���͵�CO2ˮ��Һ�Ⱥ����𰸣��� û��
��
 �������𰸺������ɣ�
�������𰸺������ɣ���3��[������]
�ٹ���
�ھ��ã����ϲ���Һ�м����μ�BaCl2��Һ��������˵��BaCl2��Һ�ѹ���
�۳���δϴ�Ӹɾ�����������ﲻ��֣�

��ϰ��ϵ�д�
�����Ŀ
ijУ��ѧ��ȤС���ͬѧ����������֪�����������ʵ��¶Ⱥ���������¿�ת��Ϊ�����ǣ�Ϊ̽�������ڲ�ͬ�¶���ת��Ϊ�����ǵij̶ȣ�����ÿ�˶���������������ʵ�飺
����1����һ֧���Թ�ȡһ����������ˮ��ϣ��ټ���������ϡ���ᣬҡ�ȣ����ȵ�һ�����¶ȣ�ʵ������У����˿����¶Ȳ�ͬ�����ȳ���ʱ����ͬ��
����2��Ȼ������֧С�Թܸ�ȡ�������Թ��ڵķ�Ӧ����������һ֧С�Թܣ����ΪA���ڵμ�NaOH��Һ����ǿ���ԣ��ٵ���4-5��CuS04��Һ�����������У�����һ֧С�Թܣ����ΪB���ڵμ�2-3�ε�ˮ���۲첢��¼����
��1�����ڸ��˿��Ƶ��¶Ȳ�ͬ������������������������������ѧ֪ʶ�����������������ʵ��������ܱ���
��2���ڲ���2��A�Թ�������Na0H��Һ�������� ��
����1����һ֧���Թ�ȡһ����������ˮ��ϣ��ټ���������ϡ���ᣬҡ�ȣ����ȵ�һ�����¶ȣ�ʵ������У����˿����¶Ȳ�ͬ�����ȳ���ʱ����ͬ��
����2��Ȼ������֧С�Թܸ�ȡ�������Թ��ڵķ�Ӧ����������һ֧С�Թܣ����ΪA���ڵμ�NaOH��Һ����ǿ���ԣ��ٵ���4-5��CuS04��Һ�����������У�����һ֧С�Թܣ����ΪB���ڵμ�2-3�ε�ˮ���۲첢��¼����
��1�����ڸ��˿��Ƶ��¶Ȳ�ͬ������������������������������ѧ֪ʶ�����������������ʵ��������ܱ���
| �� �� | �� �� | |
| ��һ�� �� �� | A�Թ��У� B�Թ��У� | ����û��ת��Ϊ������ |
| �ڶ��� �� �� | A�Թ��У� B�Թ��У� | |
| ������ �� �� | A�Թ��У� B�Թ��У� | ����ȫ��ת��Ϊ������ |
ijУ��ѧ��ȤС���ͬѧ����������֪�����������ʵ��¶Ⱥ���������¿�ת��Ϊ�����ǣ�Ϊ̽�������ڲ�ͬ�¶���ת��Ϊ�����ǵij̶ȣ�����ÿ�˶���������������ʵ�飺
����1����һ֧���Թ�ȡһ����������ˮ��ϣ��ټ���������ϡ���ᣬҡ�ȣ����ȵ�һ�����¶ȣ�ʵ������У����˿����¶Ȳ�ͬ�����ȳ���ʱ����ͬ��
����2��Ȼ������֧С�Թܸ�ȡ�������Թ��ڵķ�Ӧ����������һ֧С�Թܣ����ΪA���ڵμ�NaOH��Һ����ǿ���ԣ��ٵ���4-5��CuS04��Һ�����������У�����һ֧С�Թܣ����ΪB���ڵμ�2-3�ε�ˮ���۲첢��¼����
��1�����ڸ��˿��Ƶ��¶Ȳ�ͬ������������������������������ѧ֪ʶ�����������������ʵ��������ܱ���
��2���ڲ���2��A�Թ�������Na0H��Һ�������� ��
����1����һ֧���Թ�ȡһ����������ˮ��ϣ��ټ���������ϡ���ᣬҡ�ȣ����ȵ�һ�����¶ȣ�ʵ������У����˿����¶Ȳ�ͬ�����ȳ���ʱ����ͬ��
����2��Ȼ������֧С�Թܸ�ȡ�������Թ��ڵķ�Ӧ����������һ֧С�Թܣ����ΪA���ڵμ�NaOH��Һ����ǿ���ԣ��ٵ���4-5��CuS04��Һ�����������У�����һ֧С�Թܣ����ΪB���ڵμ�2-3�ε�ˮ���۲첢��¼����
��1�����ڸ��˿��Ƶ��¶Ȳ�ͬ������������������������������ѧ֪ʶ�����������������ʵ��������ܱ���
| �� �� | �� �� | |
| ��һ�� �� �� | A�Թ��У� B�Թ��У� | ����û��ת��Ϊ������ |
| �ڶ��� �� �� | A�Թ��У� B�Թ��У� | |
| ������ �� �� | A�Թ��У� B�Թ��У� | ����ȫ��ת��Ϊ������ |
