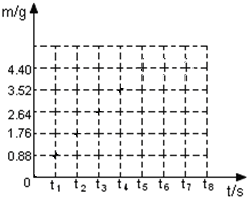
��Ŀ����
С��ͬѧ��13.9g�����ʵĴ�����Ʒ��̼�������Ȼ��ƵĻ�����90.5gϡ�������ϣ���ַ�Ӧ����÷�Ӧ���������������m���뷴Ӧʱ�䣨t�����������±���ʾ��| ��Ӧʱ��t/s | t0 | t1 | t2 | t3 | t4 | t5 | t6 |
| ��������m/g | 0 | 0.88 | 1.76 | 2.64 | 3.52 | 4.4 | 4.4 |
��1��̼������ȫ��Ӧ������CO2������Ϊ
��2���������������ͼ�У�������Ӧʱ���������������m����ʱ�䣨t���仯�����ߣ�
��3������ȫ��Ӧ��������Һ�����ʵ�������������Na2CO3+2HCl=2NaCl+CO2��+H2O����
��������1������̼���ƺ�ϡ���ᷴӦ�ܲ���������̼������ͼ�����ݷ����ó����ɶ�����̼��������
��2������ͼ�����ݺ�����ͼ�к���������ı�ʾ����Ҫ����Ӧ���������������m����ʱ�䣨t���仯�����ߣ�
��3�������ɶ�����̼����������̼������ϡ���ᷴӦ�Ļ�ѧ����ʽ���Լ������Ʒ��̼���Ƶ������������Ȼ��Ƶ������������������Ʒ���Ȼ��Ƶ��������ټ��������Ȼ��Ƶ��������Ƿ�Ӧ����Һ�е����������������������Һ�����������������������ļ��㹫ʽ���Լ������Ӧ�����Һ�����ʵ�����������
��2������ͼ�����ݺ�����ͼ�к���������ı�ʾ����Ҫ����Ӧ���������������m����ʱ�䣨t���仯�����ߣ�
��3�������ɶ�����̼����������̼������ϡ���ᷴӦ�Ļ�ѧ����ʽ���Լ������Ʒ��̼���Ƶ������������Ȼ��Ƶ������������������Ʒ���Ȼ��Ƶ��������ټ��������Ȼ��Ƶ��������Ƿ�Ӧ����Һ�е����������������������Һ�����������������������ļ��㹫ʽ���Լ������Ӧ�����Һ�����ʵ�����������
����⣺��1������ͼ�����ݷ���̼������ȫ��Ӧ������CO2������Ϊ��4.4g
��2������ͼ�����ݣ�������Ӧ���������������m����ʱ�䣨t���仯�����ߣ�
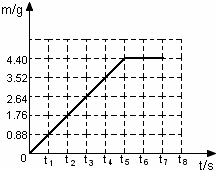
��3������Ʒ��̼���Ƶ�����Ϊx����Ӧ����NaCl������Ϊy��
Na2CO3+2HCl=2NaCl+CO2��+H2O
106 117 44
x y 4.4g
��֮�ã�x=10.6g y=11.7g
����Ʒ���Ȼ��Ƶ�����Ϊ13.9-10.6=3.3��g��
��Ӧ����Һ�����ʵ�����=11.7+3.3=15��g��
��Ӧ����Һ������=13.9+90.5-4.4=100��g��
����ȫ��Ӧ��������Һ�����ʵ���������Ϊ
��100%=15%
����ȫ��Ӧ��������Һ�����ʵ���������Ϊ15%
��2������ͼ�����ݣ�������Ӧ���������������m����ʱ�䣨t���仯�����ߣ�

��3������Ʒ��̼���Ƶ�����Ϊx����Ӧ����NaCl������Ϊy��
Na2CO3+2HCl=2NaCl+CO2��+H2O
106 117 44
x y 4.4g
��֮�ã�x=10.6g y=11.7g
����Ʒ���Ȼ��Ƶ�����Ϊ13.9-10.6=3.3��g��
��Ӧ����Һ�����ʵ�����=11.7+3.3=15��g��
��Ӧ����Һ������=13.9+90.5-4.4=100��g��
����ȫ��Ӧ��������Һ�����ʵ���������Ϊ
| 15g |
| 100g |
����ȫ��Ӧ��������Һ�����ʵ���������Ϊ15%
������������Ҫ�����й���Ʒ�����Ļ�ѧ����ʽ�ļ�����й��������������ļ��㣬�ܶ���ѧ��������˼ά�������������ѶȽϴ�

��ϰ��ϵ�д�
 ǧ�������������ĩ�����Ծ�����ϵ�д�
ǧ�������������ĩ�����Ծ�����ϵ�д�
�����Ŀ
С��ͬѧ��13.9g�����ʵĵĴ�����Ʒ��̼�������Ȼ��ƵĻ�����90.5gϡ�������ϣ���ַ�Ӧ����÷�Ӧ���������������m���뷴Ӧʱ�䣨t�����������±���ʾ��
| ��Ӧʱ��t/s | t0 | t1 | t2 | t3 | t4 | t5 | t6 |
| ��������m/g | 0 | 0.88 | 1.76 | 2.64 | 3.52 | 4.4 | 4.4 |
������ĿҪ�ش��������⣺
��1��̼������ȫ��Ӧ������CO2������Ϊ g
��2���������������ͼ�У�������Ӧ�����������������m����ʱ�䣨t���仯�����ߡ�
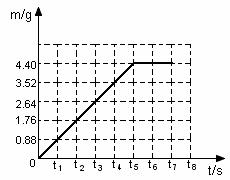
��3������ȫ��Ӧ��������Һ�����ʵ�������������Na2CO3��2HCl��2NaCl��CO2����H2O����