��Ŀ����
����һƿ��ǩ��ͼ��ʾ��Ũ���ᣬ����ݱ�ǩ�ϵ����ݻش����⣺
| ���� �����500mL ��ѧʽ��HCl ��Է���������36.5 �ܶȣ�1.1g/cm3 ����������30% |
��2�����ø����Ƶ�ϡ�������ⶨij̼������Ʒ�Ĵ��ȣ�������Ԫ�أ���ȡһ������̼���Σ�R2C03����Ʒ�������е������õ�ϡ���������ٲ�������Ϊֹ������ȥ����73g�����ʲ������ᷴӦҲ������ˮ����Ȼ����ˣ�������2.8g����������Һ���ɣ��õ����崿����23.4g��
�ٷ�����Ӧ�Ļ�ѧ����ʽ��______�ڸ���Ʒ����Ҫ�ɷֻ�ѧʽ����______
��������֪�������μӷ�Ӧ�Ĺ�������������x���ı���ʽ______
�ܸ���Ʒ�Ĵ�����______
������Ӧ����Һ�м���31.6gˮ��ʱ������Һ�����ʵ���������Ϊ______��
�⣺��1�������ø�Ũ��������Ϊx
165g��20%=x?1.1g/cm3?30%
��֮�� x=100mL
�ʴ����ø�Ũ����100mL��
��2����̼������ϡ���������Ȼ��ˮ�Ͷ�����̼������̼���εĻ�ѧʽR2CO3���ɵ�֪Ԫ��R�Ļ��ϼ�Ϊ+2�ۣ�
�ʴ�R2CO3+2HCl=2RCl+CO2��+H2O��
����RԪ�ص����ԭ������Ϊm
R2CO3+2HCl=2RCl+CO2��+H2O
73 2m+71
73g��20% 23.4g
73����2m+71��=��73g��20%����23.4g
��֮�� m=23
�������ԭ�������ɲ��RԪ��Ϊ�ƣ�Na��
�ʴ�Na2CO3
��Na2CO3+2HCl=2NaCl+CO2��+H2O
106 73
x 73g��20%
106��73=x����73g��20%��
�ʴ�106��73=x����73g��20%��
�ܹ�����̼��������x=21.2g
��Ʒ�Ĵ���= ��88.3%
��88.3%
�ʴ�88.3%
���跴Ӧ���ɶ�����̼����Ϊy
Na2CO3+2HCl=2NaCl+CO2��+H2O
73 44
73g��20% y
73��44=��73g��20%����y y=8.8g
����31.6gˮ��ʱ������Һ�����ʵ���������= =20%
=20%
�ʴ�20%
��������1���ɱ�ǩ��֪����Һ����������Ϊ30%����ˮϡ��Ϊ20%��ϡ���ᣬ��ˮǰ�������������䣻
��2��̼��������ų�������̼�����ݷ�Ӧ�е�����ȷ����Ӧ������ĩ�Ĵ��ȼ�������Һ����������������
���������������غ㶨�ɣ�����31.6gˮ��������Һ������=21.2g+73g+31.6g-8.8g=117g��
165g��20%=x?1.1g/cm3?30%
��֮�� x=100mL
�ʴ����ø�Ũ����100mL��
��2����̼������ϡ���������Ȼ��ˮ�Ͷ�����̼������̼���εĻ�ѧʽR2CO3���ɵ�֪Ԫ��R�Ļ��ϼ�Ϊ+2�ۣ�
�ʴ�R2CO3+2HCl=2RCl+CO2��+H2O��
����RԪ�ص����ԭ������Ϊm
R2CO3+2HCl=2RCl+CO2��+H2O
73 2m+71
73g��20% 23.4g
73����2m+71��=��73g��20%����23.4g
��֮�� m=23
�������ԭ�������ɲ��RԪ��Ϊ�ƣ�Na��
�ʴ�Na2CO3
��Na2CO3+2HCl=2NaCl+CO2��+H2O
106 73
x 73g��20%
106��73=x����73g��20%��
�ʴ�106��73=x����73g��20%��
�ܹ�����̼��������x=21.2g
��Ʒ�Ĵ���=
 ��88.3%
��88.3%�ʴ�88.3%
���跴Ӧ���ɶ�����̼����Ϊy
Na2CO3+2HCl=2NaCl+CO2��+H2O
73 44
73g��20% y
73��44=��73g��20%����y y=8.8g
����31.6gˮ��ʱ������Һ�����ʵ���������=
 =20%
=20%�ʴ�20%
��������1���ɱ�ǩ��֪����Һ����������Ϊ30%����ˮϡ��Ϊ20%��ϡ���ᣬ��ˮǰ�������������䣻
��2��̼��������ų�������̼�����ݷ�Ӧ�е�����ȷ����Ӧ������ĩ�Ĵ��ȼ�������Һ����������������
���������������غ㶨�ɣ�����31.6gˮ��������Һ������=21.2g+73g+31.6g-8.8g=117g��

��ϰ��ϵ�д�
�����Ŀ
����һƿ��ǩ��ͼ��ʾ��Ũ���ᣬ����ݱ�ǩ�ϵ����ݻش����⣺
��1��Ҫ����165g��20%�����ᣬ���ø�Ũ���� mL��
��2�����ø����Ƶ�ϡ�������ⶨij̼������Ʒ�Ĵ��ȣ�������Ԫ�أ���ȡһ������̼���Σ�R2C03����Ʒ�������е������õ�ϡ���������ٲ�������Ϊֹ������ȥ����73g�����ʲ������ᷴӦҲ������ˮ����Ȼ����ˣ�������2.8g����������Һ���ɣ��õ����崿����23.4g��
�ٷ�����Ӧ�Ļ�ѧ����ʽ�� �ڸ���Ʒ����Ҫ�ɷֻ�ѧʽ����
��������֪�������μӷ�Ӧ�Ĺ�������������x���ı���ʽ
�ܸ���Ʒ�Ĵ�����
������Ӧ����Һ�м���31.6gˮ��ʱ������Һ�����ʵ���������Ϊ ��
| ���� �����500mL ��ѧʽ��HCl ��Է���������36.5 �ܶȣ�1.1g/cm3 ����������30% |
��2�����ø����Ƶ�ϡ�������ⶨij̼������Ʒ�Ĵ��ȣ�������Ԫ�أ���ȡһ������̼���Σ�R2C03����Ʒ�������е������õ�ϡ���������ٲ�������Ϊֹ������ȥ����73g�����ʲ������ᷴӦҲ������ˮ����Ȼ����ˣ�������2.8g����������Һ���ɣ��õ����崿����23.4g��
�ٷ�����Ӧ�Ļ�ѧ����ʽ��
��������֪�������μӷ�Ӧ�Ĺ�������������x���ı���ʽ
�ܸ���Ʒ�Ĵ�����
������Ӧ����Һ�м���31.6gˮ��ʱ������Һ�����ʵ���������Ϊ
����һƿ��ǩ��ͼ��ʾ��Ũ���ᣬ����ݱ�ǩ�ϵ����ݻش����⣺
| ���� �����500mL ��ѧʽ��HCl ��Է���������36.5 �ܶȣ�1.2g/cm3 ����������37%��1���ø�Ũ����100ml��������������Ϊ20%������ ��2�����ø����Ƶ�ϡ�������ⶨij̼������Ʒ�Ĵ��ȣ���Ʒ������Ԫ�أ���ȡ50g����Ʒ�������е������õ�ϡ���������ٲ�������Ϊֹ������ȥ����146g�� �ٷ�����Ӧ�Ļ�ѧ����ʽ ��������֪�������μӷ�Ӧ�Ĺ�������������x���ı���ʽ �۸���Ʒ�ijɷ��� �ܸ���Ʒ�Ĵ����� ������Ӧ����Һ�м���46.8gˮ��ʱ������Һ�����ʵ���������Ϊ |
 ����һƿ��ǩ��ͼ��ʾ��Ũ���ᣬ����ݱ�ǩ�ϵ����ݻش����⣺
����һƿ��ǩ��ͼ��ʾ��Ũ���ᣬ����ݱ�ǩ�ϵ����ݻش����⣺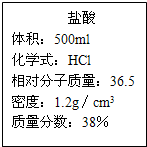 ����һƿ��ǩ��ͼ��ʾ��Ũ���ᣬ����ݱ�ǩ����������⣺
����һƿ��ǩ��ͼ��ʾ��Ũ���ᣬ����ݱ�ǩ����������⣺ ��1����������炙�ѧʽΪNH4NO3������NԪ�صĻ��ϼ�Ϊ
��1����������炙�ѧʽΪNH4NO3������NԪ�صĻ��ϼ�Ϊ