��Ŀ����
ʵ���ҳ���ʯ��ʯ��ϡ��������µ�ijЩװ������ȡ���ռ�������̼��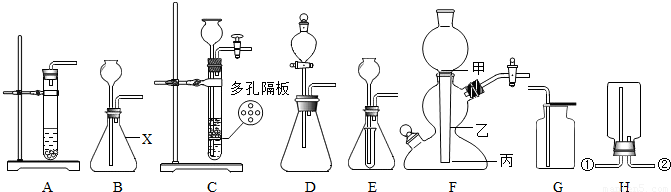
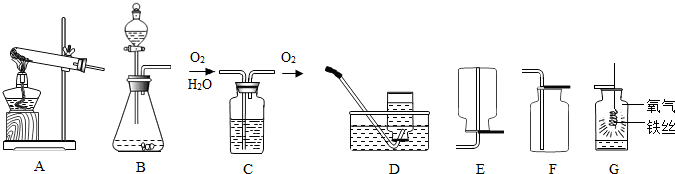
��1��ʵ����ͨ����ͼA��ͼB��װ������ȡ������̼��ͼBװ��������X�������� �����ͼBװ�õ������Եķ����� ��
��2����ͼAװ�ý��иĽ��ɵ�ͼCװ�ã����ͼAװ�ã�ͼCװ�õ���Ҫ�ŵ��DZ�������ϡ����� ��
��3����ͼBװ�ý��иĽ��ɵ�ͼDװ�û�ͼEװ�ã�ͼEװ����С�Թܵ������� ��
��4����Ҫ�������ȡ����������̼����õ���ͼFװ��-���շ�������ʵ�����У�ʯ��ʯ����ͼFװ���� ��λ��ѡ��ס��һ������
��5��ͨ������¶�����̼�ܶȴ��ڿ������ʳ���ͼGװ�����ռ�������̼������ͼHװ�����ռ�������̼������Ӧ�ӵ��ܿ� ��ѡ��ٻ�ڣ�ͨ�룮������̼��Ȼ������ˮ��������ˮ�е��ݳ����ʴ��� ���ʣ���Ҳ������ˮ���ռ���
��6��ʵ��ʱ����ʢ��ʯ��ʯ���Թ��м�������ϡ�������ʯ��ʯ������м��������ݲ��������ܵ�ԭ���� ��дһ�֣���
��7����ʵ�������Ʊ�2.2L CO2�����������贿��Ϊ80%��ʯ��ʯ�������� �������״̬��CO2���ܶ�Ϊ2g��L-1����
���𰸡���������1��ʵ�����г���ʯ��ʯ��ϡ������ȡ������̼������װ�������������Ƽ����ã����װ��B�����ԣ�����������裺�õ��ɼм�ס�ܣ���©����ע��ˮ��������©�����γ�һ���ȶ���ˮ����˵��װ�ò�©����
��2���Ľ����Fװ���е��п����ϰ���ѿ�״����ʯ��ʯ��ϡ������뿪����Ҫ������Ӧʱʹ����Ӧ��Ӵ���������Ӧ������Ҫʱ����Ʋ����������ų���ʹ����ѹǿ���ϡ����ѹ�볤��©����ʹ��Ӧ������Ӵ����ﵽ�˿��Ʒ�Ӧ������ֹͣ�����ã�
��3������ʹ�ó���©����ע���������С�Թܵ����ã�
��4���������շ������Ľṹ�����÷�����Һ��λ�ã�
��5�����ݶ�����̼���ܶ�ѡ������ڣ����ݶ�����̼��ˮ��Ӧ����̼�ᣬ��̼��ȶ�������
��6���ӷ�Ӧ��ĽǶȷ�������������̼�ٵ�ԭ��
��7�������ȶ�����̼�������������������û�ѧ����ʽ���г�����ʽ�������̼��Ƶ�������Ȼ�����������������ʯ��ʯ��������
����⣺��1��ʵ�����г���ʯ��ʯ��ϡ������ȡ������̼������X����������ƿ�����װ��B�����Ծ���������裺�õ��ɼм�ס�ܣ���©����ע��ˮ��������©�����γ�һ���ȶ���ˮ����˵��װ�ò�©����
�ʴ�Ϊ����ƿ���õ��ɼм�ס�ܣ���©����ע��ˮ��������©�����γ�һ���ȶ���ˮ����˵��װ�ò�©����
��2����״����ʯ��ʯ���ڸ����ϣ�ͨ�������Ŀ��ؿ��Կ���ϡ������ʯ��ʯ�ĽӴ������룬��Ӧ��Ӵ�ʱ��Ӧ��������Ӧ������Ӵ�ʱ��Ӧֹͣ��
�ʴ�Ϊ��������ʱ���Ʒ�Ӧ�ķ�����ֹͣ��
��3��ʹ�ó���©��ʱҪ��©����ĩ�˱�������Һ�����£����������ӳ���©������������ͼEװ����С�Թܵ��������γ�Һ�⣬��ֹ���ɵ�������ɢ�������У�Ҳ���Խ�ԼҩƷ��
�ʴ�Ϊ���γ�Һ�⣬��ֹ���ɵ�������ɢ�������У�
��4��������������շ��������Ҵ���Һ��Ӽ�����������Ҵ��Ĺ���Ӵ�����Ӧ������
�ʴ�Ϊ���ң�
��5����Ϊ������̼���ܶȱȿ����ʶ�����̼Ӧ�þۼ��ڼ���ƿ�ĵײ����ʽ������Ƕ̹ܢڣ�������̼��Ȼ������ˮ����ˮ��Ӧ����̼�ᣬ��̼��ȶ����ֽ��������̼����������ˮ�е��ݳ����ʴ������ܽ⣨����ˮ��Ӧ�����ʣ���Ҳ������ˮ���ռ���
�ʴ�Ϊ���ڣ��ܽ⣨����ˮ��Ӧ����
��6��ʵ��ʱ����ʢ��ʯ��ʯ���Թ��м�������ϡ�������ʯ��ʯ������м��������ݲ�����ԭ�������ʯ��ʯ��̼��Ƶĺ������ͻ�ϡ�����Ũ�ȹ��ͣ�
�ʴ�Ϊ��ʯ��ʯ��̼��Ƶĺ������ͻ�ϡ�����Ũ�ȹ��ͣ�
��7���⣺��̼��Ƶ�����Ϊx
m��CO2��=2g/L×2.2L=4.4g
CaC03+2HCl�TCaCl2+CO2��+H2
100 44
x 4.4g
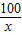 =
=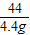
x=10g
ʯ��ʯ������Ϊ10g÷80%=12.5g
���������贿��Ϊ80%��ʯ��ʯ12.5g��
�����������ۺϿ�����ʵ������ȡ������̼��װ���ص�������ݻ�ѧ����ʽ���еļ��㣬Ҫ��������֪ʶ����������ã�ֻ��������������ȷ�������Ŀ��
��2���Ľ����Fװ���е��п����ϰ���ѿ�״����ʯ��ʯ��ϡ������뿪����Ҫ������Ӧʱʹ����Ӧ��Ӵ���������Ӧ������Ҫʱ����Ʋ����������ų���ʹ����ѹǿ���ϡ����ѹ�볤��©����ʹ��Ӧ������Ӵ����ﵽ�˿��Ʒ�Ӧ������ֹͣ�����ã�
��3������ʹ�ó���©����ע���������С�Թܵ����ã�
��4���������շ������Ľṹ�����÷�����Һ��λ�ã�
��5�����ݶ�����̼���ܶ�ѡ������ڣ����ݶ�����̼��ˮ��Ӧ����̼�ᣬ��̼��ȶ�������
��6���ӷ�Ӧ��ĽǶȷ�������������̼�ٵ�ԭ��
��7�������ȶ�����̼�������������������û�ѧ����ʽ���г�����ʽ�������̼��Ƶ�������Ȼ�����������������ʯ��ʯ��������
����⣺��1��ʵ�����г���ʯ��ʯ��ϡ������ȡ������̼������X����������ƿ�����װ��B�����Ծ���������裺�õ��ɼм�ס�ܣ���©����ע��ˮ��������©�����γ�һ���ȶ���ˮ����˵��װ�ò�©����
�ʴ�Ϊ����ƿ���õ��ɼм�ס�ܣ���©����ע��ˮ��������©�����γ�һ���ȶ���ˮ����˵��װ�ò�©����
��2����״����ʯ��ʯ���ڸ����ϣ�ͨ�������Ŀ��ؿ��Կ���ϡ������ʯ��ʯ�ĽӴ������룬��Ӧ��Ӵ�ʱ��Ӧ��������Ӧ������Ӵ�ʱ��Ӧֹͣ��
�ʴ�Ϊ��������ʱ���Ʒ�Ӧ�ķ�����ֹͣ��
��3��ʹ�ó���©��ʱҪ��©����ĩ�˱�������Һ�����£����������ӳ���©������������ͼEװ����С�Թܵ��������γ�Һ�⣬��ֹ���ɵ�������ɢ�������У�Ҳ���Խ�ԼҩƷ��
�ʴ�Ϊ���γ�Һ�⣬��ֹ���ɵ�������ɢ�������У�
��4��������������շ��������Ҵ���Һ��Ӽ�����������Ҵ��Ĺ���Ӵ�����Ӧ������
�ʴ�Ϊ���ң�
��5����Ϊ������̼���ܶȱȿ����ʶ�����̼Ӧ�þۼ��ڼ���ƿ�ĵײ����ʽ������Ƕ̹ܢڣ�������̼��Ȼ������ˮ����ˮ��Ӧ����̼�ᣬ��̼��ȶ����ֽ��������̼����������ˮ�е��ݳ����ʴ������ܽ⣨����ˮ��Ӧ�����ʣ���Ҳ������ˮ���ռ���
�ʴ�Ϊ���ڣ��ܽ⣨����ˮ��Ӧ����
��6��ʵ��ʱ����ʢ��ʯ��ʯ���Թ��м�������ϡ�������ʯ��ʯ������м��������ݲ�����ԭ�������ʯ��ʯ��̼��Ƶĺ������ͻ�ϡ�����Ũ�ȹ��ͣ�
�ʴ�Ϊ��ʯ��ʯ��̼��Ƶĺ������ͻ�ϡ�����Ũ�ȹ��ͣ�
��7���⣺��̼��Ƶ�����Ϊx
m��CO2��=2g/L×2.2L=4.4g
CaC03+2HCl�TCaCl2+CO2��+H2
100 44
x 4.4g
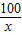 =
=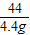
x=10g
ʯ��ʯ������Ϊ10g÷80%=12.5g
���������贿��Ϊ80%��ʯ��ʯ12.5g��
�����������ۺϿ�����ʵ������ȡ������̼��װ���ص�������ݻ�ѧ����ʽ���еļ��㣬Ҫ��������֪ʶ����������ã�ֻ��������������ȷ�������Ŀ��

��ϰ��ϵ�д�
�����Ŀ
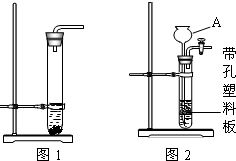 ʵ���ҳ���ʯ��ʯ��ϡ������ȡC02��ijͬѧ�����������ȡCO2�IJ���װ�ã�����ͼ����
ʵ���ҳ���ʯ��ʯ��ϡ������ȡC02��ijͬѧ�����������ȡCO2�IJ���װ�ã�����ͼ����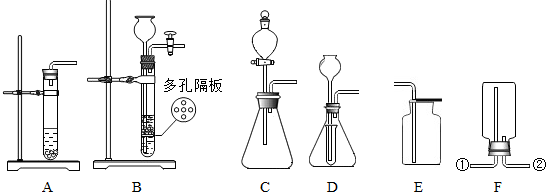
 ��2013?�ɱ�����ģ��ʵ���ҳ���ʯ��ʯ��ϡ���ᷴӦ��ȡ������̼����ش��������⣺
��2013?�ɱ�����ģ��ʵ���ҳ���ʯ��ʯ��ϡ���ᷴӦ��ȡ������̼����ش��������⣺