��Ŀ����
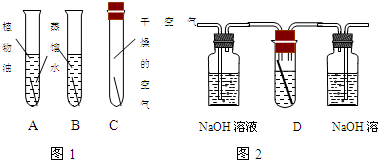
ͭ�����Ҫ�ɷ���ͭ�̣���ѧʽΪ[Cu2��OH��2CO3]��Ϊ����֤ͭ����������ijУ��ѧ��ȤС��ij�Ա����������ͼ��ʵ�飮Aͼ��Bͼ��Cͼ��ͭ����û�����⣬Dͼͭ�������⣮��1��ͭ��������Ҫ������ʵ������ͭ��______��______��______����ã�������ѧ��Ӧ�Ľ����
��2��д��Cͼ���Լ�ƿ����������Ӧ�Ļ�ѧ����ʽ______
��3��ͭ������ȣ�Fe�������⣬�ɴ˿ɵó��Ľ�����______��
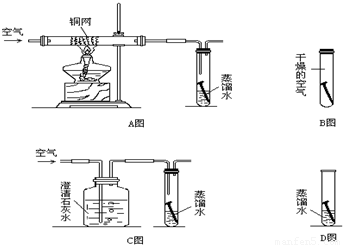
���𰸡�����������ͭ������������з�����ͭ�������ͭ��������ˮ�Ͷ�����̼���棬������̼�����������Ʒ�Ӧ����̼��ƺ�ˮ������ͭ�����⣬˵�����Ļ�Ա�ͭǿ��
����⣺��1��Aͼ�г�ȥ����ͭ�����⣬Bͼ�г�ȥˮ����ͭ�����⣬Cͼ�г�ȥ������̼ͭ�����⣬˵��ͭ������ͭ��������ˮ�Ͷ�����̼��ͬ���õĽ�������������ˮ��������̼��
��2��������̼�����������Ʒ�Ӧ����̼��ƺ�ˮ�����CO2+Ca��OH��2�TCaCO3��+H2O��
��3��ͭ������ȣ�Fe�������⣬˵�����Ļ�Ա�ͭǿ��������Ļ�Ա�ͭǿ��
���������⿼����ͭ�������������ɴ��⣬������������ṩ��ʵ����з������Ӷ��ó���ȷ�Ľ��ۣ�
����⣺��1��Aͼ�г�ȥ����ͭ�����⣬Bͼ�г�ȥˮ����ͭ�����⣬Cͼ�г�ȥ������̼ͭ�����⣬˵��ͭ������ͭ��������ˮ�Ͷ�����̼��ͬ���õĽ�������������ˮ��������̼��
��2��������̼�����������Ʒ�Ӧ����̼��ƺ�ˮ�����CO2+Ca��OH��2�TCaCO3��+H2O��
��3��ͭ������ȣ�Fe�������⣬˵�����Ļ�Ա�ͭǿ��������Ļ�Ա�ͭǿ��
���������⿼����ͭ�������������ɴ��⣬������������ṩ��ʵ����з������Ӷ��ó���ȷ�Ľ��ۣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
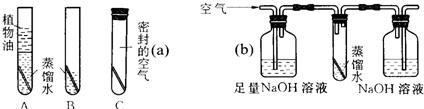

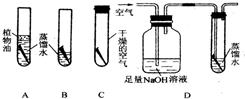 25��ͭ�����Ҫ�ɷ���ͭ�̣�ij��ѧ��ȤС��Ϊ���о�ͭ���������������������ͼ��ʾ��ʵ�飮һ���º���B�е�ͭ˿�������⣬��ˮ�洦ͭ˿�����Ϊ���أ���A��C��D�е�ͭ˿�����ޱ仯���Ը���ʵ��ش��������⣺
25��ͭ�����Ҫ�ɷ���ͭ�̣�ij��ѧ��ȤС��Ϊ���о�ͭ���������������������ͼ��ʾ��ʵ�飮һ���º���B�е�ͭ˿�������⣬��ˮ�洦ͭ˿�����Ϊ���أ���A��C��D�е�ͭ˿�����ޱ仯���Ը���ʵ��ش��������⣺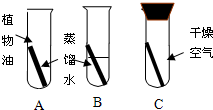 ��2005?������һģ����B�飩ͭ�����Ҫ�ɷ���ͭ��[Cu2��OH��2CO3]��ijͬѧΪ��̽��ͭƬ�ڿ����������ԭ��������ͼ��ʾA��B��Cװ�ý���ʵ�飬������һ���µĹ۲죬��ͬѧ�ᷢ��
��2005?������һģ����B�飩ͭ�����Ҫ�ɷ���ͭ��[Cu2��OH��2CO3]��ijͬѧΪ��̽��ͭƬ�ڿ����������ԭ��������ͼ��ʾA��B��Cװ�ý���ʵ�飬������һ���µĹ۲죬��ͬѧ�ᷢ��