��Ŀ����
Сǿͬѧ�ڻ�ѧʵ���ҷ���һƿʢ�а�ɫ��ĩ���Լ�ƿ�����ǩ����������ʦ����������ƿ�Լ������������ơ�̼���ơ�̼�������е�һ�֣�Сǿͬѧͨ���������ϵ�֪�����������������ʵ��ܽ�����±���
| ���� | Na2SO4 | Na2CO3 | NaHCO3 |
| �ܽ��/g | 19.5 | 21.5 | 9.6 |
��2��Ϊȷ����ɷ֣�Сǿ��ͬѧ�Դ���Һ������������ʵ��̽����
| ʵ���顡������ | ʵ���顡�֡��� | ʵ���顡�ᡡ�� |
| ȡ������Һ���Թ��У���ϡ���� | ��Һ�����ݲ��� | ����Һ��________��Һ |
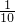 ������������������Һ��Ӧ���������ܲ������ٿ˳�����
������������������Һ��Ӧ���������ܲ������ٿ˳������� �õ��Ļ�ѧ����ʽ��Na2CO3+Ba��OH��2�TBaCO3��+2NaOH ��
�⣺��1���������������ڳ����µ��ܽ�ȿɿ�����ֻ��̼�����Ƶ��ܽ��С��10.6g������ȫ���ܽ⣻��Ϊ����Һ�DZ�����Һ�����ݱ�����Һ���������������ļ���ɼ��������Һ��������������Ϊ ��100%=9.6%�ʴ�Ϊ��NaHCO3��9.6%
��100%=9.6%�ʴ�Ϊ��NaHCO3��9.6%
��2������̼���ƣ��ɸ���̼�����������ᷴӦ���ɶ�����̼��������ʣ�ȡ������ĩ����ϡ���ᣬ���Ƿ����������壮������ȡ������Һ���Թ��У���ϡ���ᣬ��Һ�����ݲ�����˵������Һ��̼������Һ���ʴ�Ϊ��Na2CO3
��3�������ɳ���������ΪX
Na2CO3+Ba��OH��2�TBaCO3��+2NaOH
106 197
10.6g��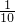 x
x
 =
=
x=1.97g
���������ܲ���������������1.97�ˣ�
��������1���������ʵ��ܽ���жϣ�
��2���ɸ���̼���������ᷴӦ���ɶ�����̼���������
��3�����ݻ�ѧ����ʽ���м��㣬ע�����IJ��裮����Na2CO3+Ba��OH��2�TBaCO3��+2NaOH ���ҳ�̼���ƺ�̼�ᱵ�������ȣ�Ȼ�����̼���Ƶ����������������������
������������Ҫ�������ܽ�ȵĸ��̼���Ƶļ����Լ����ݻ�ѧ����ʽ�ļ�����й�֪ʶ��ͨ��ѧ���ڼ���ʱ�����������ʵ�ʲμӷ�Ӧ������Һ�����ʵ����������Ҫ��ѧ���㹻ϸ�ġ��������������������
 ��100%=9.6%�ʴ�Ϊ��NaHCO3��9.6%
��100%=9.6%�ʴ�Ϊ��NaHCO3��9.6%��2������̼���ƣ��ɸ���̼�����������ᷴӦ���ɶ�����̼��������ʣ�ȡ������ĩ����ϡ���ᣬ���Ƿ����������壮������ȡ������Һ���Թ��У���ϡ���ᣬ��Һ�����ݲ�����˵������Һ��̼������Һ���ʴ�Ϊ��Na2CO3
��3�������ɳ���������ΪX
Na2CO3+Ba��OH��2�TBaCO3��+2NaOH
106 197
10.6g��
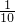 x
x =
=
x=1.97g
���������ܲ���������������1.97�ˣ�
��������1���������ʵ��ܽ���жϣ�
��2���ɸ���̼���������ᷴӦ���ɶ�����̼���������
��3�����ݻ�ѧ����ʽ���м��㣬ע�����IJ��裮����Na2CO3+Ba��OH��2�TBaCO3��+2NaOH ���ҳ�̼���ƺ�̼�ᱵ�������ȣ�Ȼ�����̼���Ƶ����������������������
������������Ҫ�������ܽ�ȵĸ��̼���Ƶļ����Լ����ݻ�ѧ����ʽ�ļ�����й�֪ʶ��ͨ��ѧ���ڼ���ʱ�����������ʵ�ʲμӷ�Ӧ������Һ�����ʵ����������Ҫ��ѧ���㹻ϸ�ġ��������������������

��ϰ��ϵ�д�
�����Ŀ
Сǿͬѧ�ڻ�ѧʵ���ҷ���һƿʢ�а�ɫ��ĩ���Լ�ƿ�����ǩ����������ʦ����������ƿ�Լ������������ơ�̼���ơ�̼�������е�һ�֣�Сǿͬѧͨ���������ϵ�֪�����������������ʵ��ܽ�����±���
��1��Сǿͬѧ�ڳ����°�10.6g�İ�ɫ��ĩ����100gˮ�У����ַ�ĩȫ���ܽ⣮����������Ϣ��������Ϊ����Һһ������______��Һ������Һ��������������Ϊ______��
��2��Ϊȷ����ɷ֣�Сǿ��ͬѧ�Դ���Һ������������ʵ��̽����
��3��Сǿͬѧȡ��1�����Ƶ���Һ�� ������������������Һ��Ӧ���������ܲ������ٿ˳�����
������������������Һ��Ӧ���������ܲ������ٿ˳�����
�� �õ��Ļ�ѧ����ʽ��Na2CO3+Ba��OH��2�TBaCO3��+2NaOH ��
| ���� | Na2SO4 | Na2CO3 | NaHCO3 |
| �ܽ��/g | 19.5 | 21.5 | 9.6 |
��2��Ϊȷ����ɷ֣�Сǿ��ͬѧ�Դ���Һ������������ʵ��̽����
| ʵ �� �� �� | ʵ �� �� �� | ʵ �� �� �� |
| ȡ������Һ���Թ��У���ϡ���� | ��Һ�����ݲ��� | ����Һ��______��Һ |
 ������������������Һ��Ӧ���������ܲ������ٿ˳�����
������������������Һ��Ӧ���������ܲ������ٿ˳������� �õ��Ļ�ѧ����ʽ��Na2CO3+Ba��OH��2�TBaCO3��+2NaOH ��
Сǿͬѧ�ڻ�ѧʵ���ҷ���һƿʢ����ɫ��Һ���Լ�ƿ�����ǩ����������ͼ��ʾ������ʦ����������ƿ�Լ����������ᡢ�����ơ�����þ��Һ�е�һ�֣�Сǿͬѧͨ���������ϵ�֪�����������������ʵ��ܽ�����±���
��1��Сǿͬѧ����������Ϣ��������Ϊ����Һһ������______��Һ����������______��
��2��Ϊȷ����ɷ֣�Сǿ��Ϊ����Һ������______��
��3������ͨ��ʵ��֤��Сǿ�IJ�������ȷ�ģ�
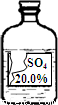
| �� �� | H2SO4 | Na2SO4 | MgSO4 |
| �ܽ��/g | ��ˮ����Ȼ��� | 19.0 | 39.0 |
��2��Ϊȷ����ɷ֣�Сǿ��Ϊ����Һ������______��
��3������ͨ��ʵ��֤��Сǿ�IJ�������ȷ�ģ�
| ʵ�鲽�� | ʵ������ | ʵ����� |
| Сǿ�IJ�������ȷ�� |

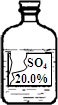 Сǿͬѧ�ڻ�ѧʵ���ҷ���һƿʢ����ɫ��Һ���Լ�ƿ�����ǩ����������ͼ��ʾ������ʦ����������ƿ�Լ����������ᡢ�����ơ�����þ��Һ�е�һ�֣�Сǿͬѧͨ���������ϵ�֪�����������������ʵ��ܽ�����±���
Сǿͬѧ�ڻ�ѧʵ���ҷ���һƿʢ����ɫ��Һ���Լ�ƿ�����ǩ����������ͼ��ʾ������ʦ����������ƿ�Լ����������ᡢ�����ơ�����þ��Һ�е�һ�֣�Сǿͬѧͨ���������ϵ�֪�����������������ʵ��ܽ�����±���
