��Ŀ����
CuSO4 �����ĩ�к���FeSO4 ���ʣ�ȡ�ù����ĩ 10g��ȫ������ˮ����� 100g��Һ�������м�����������ۣ���ַ�Ӧ����ˣ��õ���ҺA�ͳ��� B�����ⶨ����B��ֻ�����ֽ� �����ʣ������B�м���������ᣬ��ַ�Ӧ���ٹ��ˣ��ó���C������C��ϴ�ӡ������������Ϊ 3.2g�����㣺
��1��ԭ�����ĩ��CuSO4 ��������
��2����Һ A�����ʵ�������������������ȷ�� 0.1%����
�⣺��ԭ�����ĩ��CuSO4 ������Ϊx�����ɵ���������������Ϊy���μӷ�Ӧ��������Ϊz
Fe+CuSO4=FeSO4 +Cu
56 160 152 64
z x y 3.2g

x=8g y=7.6g z=2.8g
��Ӧ����Һ��������������������Ϊ��7.6g+��10g-8g��=9.6g
��Ӧ����Һ������Ϊ��100g+2.8g-3.2g=99.6g
A�����ʵ���������Ϊ��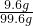 ��100%�T9.6%
��100%�T9.6%
�𣺣�1��ԭ�����ĩ��CuSO4 ������Ϊ8g��
��2����Һ A�����ʵ���������Ϊ9.6%��
��������1�����������֪������ó���C������Ϊ���ɵ�ͭ�����������ݻ�ѧ����ʽ���������ͭ��������
��2����ҺAΪ����������Һ���������ɵ�ͭ��������������ɵ�����������������Ȼ��������ĩ�к���FeSO4 ��������ҺA�����ʵ���������Һ������Ϊ��100g��Һ���ϲμӷ�Ӧ������������ȥ���ɵ�ͭ�������������������������ļ��㹫ʽ������������=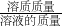 ��100%�������Һ A�����ʵ�����������
��100%�������Һ A�����ʵ�����������
������������Ҫ�����йػ�ѧ����ʽ�ļ�����������������ļ��㣬�ѶȽ�С��
Fe+CuSO4=FeSO4 +Cu
56 160 152 64
z x y 3.2g

x=8g y=7.6g z=2.8g
��Ӧ����Һ��������������������Ϊ��7.6g+��10g-8g��=9.6g
��Ӧ����Һ������Ϊ��100g+2.8g-3.2g=99.6g
A�����ʵ���������Ϊ��
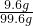 ��100%�T9.6%
��100%�T9.6%�𣺣�1��ԭ�����ĩ��CuSO4 ������Ϊ8g��
��2����Һ A�����ʵ���������Ϊ9.6%��
��������1�����������֪������ó���C������Ϊ���ɵ�ͭ�����������ݻ�ѧ����ʽ���������ͭ��������
��2����ҺAΪ����������Һ���������ɵ�ͭ��������������ɵ�����������������Ȼ��������ĩ�к���FeSO4 ��������ҺA�����ʵ���������Һ������Ϊ��100g��Һ���ϲμӷ�Ӧ������������ȥ���ɵ�ͭ�������������������������ļ��㹫ʽ������������=
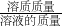 ��100%�������Һ A�����ʵ�����������
��100%�������Һ A�����ʵ�����������������������Ҫ�����йػ�ѧ����ʽ�ļ�����������������ļ��㣬�ѶȽ�С��

��ϰ��ϵ�д�
�����Ŀ