��Ŀ����
ij��ѧ��ȤС���ˮ����ͨ�����ȵ�ľ̿�õ��Ļ���������Ҫ�ɷֲ�������Ȥ��ͬѧ�Ǿ���ͨ��ʵ�����̽����
��������롿�û��������Ҫ�ɷ�Ϊһ����̼��������̼��������ˮ������
���������ϡ�a��Ũ�������������� b�������ڼ��ȵ�������������ͭ��![]() Ӧ����ͭ��ˮ��
Ӧ����ͭ��ˮ��
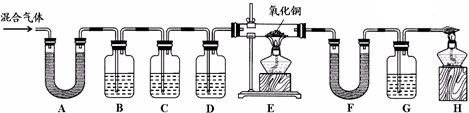
��ʵ����̡�ͬѧ������ʦ��ָ�������������ͼ��ʾװ�ã���������ʵ��(���ּг���������ȥ)��������ÿ��װ�����ҩƷ��������Ӧ��֣�
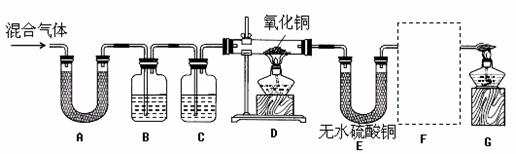
��1��װ��A����ˮ����ͭ������װ��B�г���ʯ��ˮ����ǡ��ɴ˵ó���������к�
�� ���塣д��װ��B�з�Ӧ�Ļ�ѧ����ʽ ��
��2��ͬѧ��ͨ���۲�װ��D��E�е������ ��Ϊ��ȷ�ϻ�������к�������������ȷװ��C���Լ������Ƽ���;�� ��Ϊ��֤������������Ĵ��ڣ�װ��F�е�ҩƷ������ͽ���![]() �� ��
�� ��
��ʵ����ۡ�������ȷ��
��ʵ�鷴˼���������ۣ�ͬѧ�ǽ���ͼ��װ�ý����˼��Ľ����װ������ͼ��ʾ��
��3��д��U��A�з�����Ӧ�Ļ�ѧ����ʽ ��
| ʵ�鲽�� | ʵ������ | ʵ����� | |
| �٣�19�� | �ڼ��쵼�ܴ���ȼ���壬 �� |
| �� |
| �ڣ�20�� | ��
|
|
|
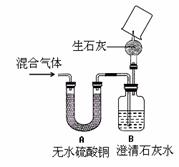 ��4��ͬѧ��Ϊ����֤ͨ������ܺ�����ijɷ֣���������
��4��ͬѧ��Ϊ����֤ͨ������ܺ�����ijɷ֣���������![]() ��ʵ��Ϊ��
��ʵ��Ϊ��
ˮ����(��H2O)��������̼(��CO2)��
Ca(OH)2+CO2 ��CaCO3�� + H2O
Ũ���� ����ˮ�� ����ʯ��ˮ������ǣ�������ɫ��������һ����̼���塣
CuSO4 + 5H2O��CuSO4��5H2O
| ���� | �� | �� |
| ʵ����� | ʵ������ | ʵ����� |
| �ڼ��쵼�ܴ���ȼ���壬 �ڻ����Ϸ���һֻ������ձ� �� | �ձ��ڱ���ˮ�� �� | ԭ��������������� �� |
| �� |
|
|
| �������ձ���Ѹ�ٵ�����������ʯ��ˮ���� �����ڻ����Ϸ���һֻ�ڱ�Ϳ�г���ʯ��ˮ���ձ��� �� | ����ʯ��ˮ����ǣ�������ɫ���� | ԭ�����������һ����̼ |

 �Ķ��쳵ϵ�д�
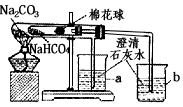
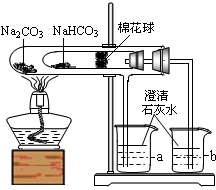
�Ķ��쳵ϵ�д�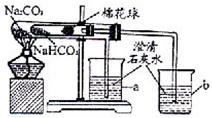 27��ij��ѧ��ȤС���ͬѧ��ѧϰ�˴��Na2CO3�������ʺ����뵽����������ͷʱ���õ�����С�մ�NaHCO3�������Dz������۵�С�մ���Ʒ����������ʵ��̽����
27��ij��ѧ��ȤС���ͬѧ��ѧϰ�˴��Na2CO3�������ʺ����뵽����������ͷʱ���õ�����С�մ�NaHCO3�������Dz������۵�С�մ���Ʒ����������ʵ��̽����