��Ŀ����
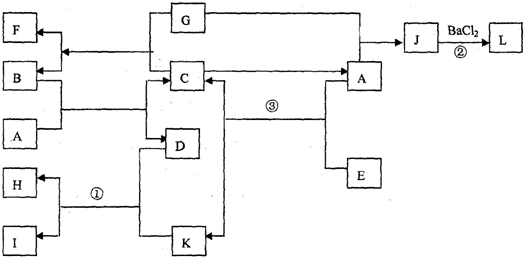
����A��H����ͼת����ϵ����Ӧ����������ȥ������B��E������Ϊ���嵥�ʣ�D�ڳ���������ɫҺ�壬C������Ϊ���壬����C�������˹����꣬F����Է�������Ϊ100�Ҳ�����ˮ�İ�ɫ���壮��ش����������⣺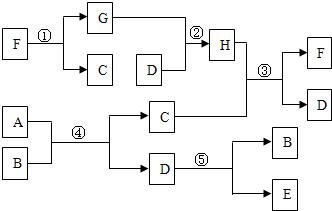
��1������ת�������ڻ��Ϸ�Ӧ����
��2��G�Ļ�ѧʽΪ
��3����Ӧ�ݵĻ�ѧ����ʽΪ
��4��Ϊȷ��A�Ļ�ѧʽ������Ϊ������Ҫ�ⶨ������Щ���ݣ�
a���μӷ�Ӧ��A������ b���μӷ�Ӧ��B������ c������C������
d������D������ e��A����Է������� f����Ӧ�ܷ���ʱ���¶ȣ�
���������⿴�����ܸ��ӣ����ʺܶ࣬��ʵ��ϸһ�����ܶ�ط�����������ͬ�ģ�����������������ж�����û��ȫ��չ������Ȼ������Ӱ����Ҫ�жϳ�ȫ�����ʣ��������������ж�����Ե��ƶ�ͻ�ƿڣ�������C�����壬��̬C�����˹����꣬˵��C��CO2��F��Է�������Ϊ100�IJ�����ˮ�Ĺ��壬�����Ծ���CaCO3��������������������������ƶϾ������ˣ�
����⣺
��1���������Ǻ������ҵ���Ŀ��ͻ�ƿڣ�������C�����壬��̬C�����˹����꣬˵��C��CO2��F��Է�������Ϊ100�IJ�����ˮ�Ĺ��壬�����Ծ���CaCO3��
��2��֪��F��C�ܹ��ж�G��CaO����D����ɫҺ�����ܹ���CaO��Ӧ��˵��D��H2O��Ҳ��֪��H��Ca��OH��2��
��3�������ת����ϵ�У�AB��Ӧ����CO2��H2O��ͬʱB�����嵥�ʣ�˵��BΪ������AΪ��̼�⣨���ܺ��������Ļ������Ӧ����ˮ�ֽ�ΪH2��O2��
��4������A�Ļ�ѧʽ���ж�����Ҫ��������CO2��H2O�������жϳ�̼Ԫ�غ���Ԫ�ص�������������A���ʵ������Աȣ����ж��Ƿ�����Ԫ�أ����Ҽ�����Ԫ�ص�������Ȼ�������㻯ѧʽ����ȻҲ���Ը���CO2��H2O���������̼Ԫ�غ���Ԫ��������ͬʱ���Ƴ�����������Ԫ�ص������ͣ��ͷ�Ӧ��B�������Աȣ����A�еĺ��������Ӷ������Ƶ���A�Ļ�ѧʽ�����Կ�����acdҲ������bcd
�ʴ𰸣�
��1���ڣ�
��2��CaO��
��3��2H2O
2H2��+O2����
��4��acd����bcd��
��1���������Ǻ������ҵ���Ŀ��ͻ�ƿڣ�������C�����壬��̬C�����˹����꣬˵��C��CO2��F��Է�������Ϊ100�IJ�����ˮ�Ĺ��壬�����Ծ���CaCO3��
��2��֪��F��C�ܹ��ж�G��CaO����D����ɫҺ�����ܹ���CaO��Ӧ��˵��D��H2O��Ҳ��֪��H��Ca��OH��2��
��3�������ת����ϵ�У�AB��Ӧ����CO2��H2O��ͬʱB�����嵥�ʣ�˵��BΪ������AΪ��̼�⣨���ܺ��������Ļ������Ӧ����ˮ�ֽ�ΪH2��O2��
��4������A�Ļ�ѧʽ���ж�����Ҫ��������CO2��H2O�������жϳ�̼Ԫ�غ���Ԫ�ص�������������A���ʵ������Աȣ����ж��Ƿ�����Ԫ�أ����Ҽ�����Ԫ�ص�������Ȼ�������㻯ѧʽ����ȻҲ���Ը���CO2��H2O���������̼Ԫ�غ���Ԫ��������ͬʱ���Ƴ�����������Ԫ�ص������ͣ��ͷ�Ӧ��B�������Աȣ����A�еĺ��������Ӷ������Ƶ���A�Ļ�ѧʽ�����Կ�����acdҲ������bcd
�ʴ𰸣�
��1���ڣ�
��2��CaO��
��3��2H2O
| ||
��4��acd����bcd��
������������ʽ��Ϊ���ӵ��ƶ��⣬Ҫ�����Ӿֲ�ȥ��������һͻ�ƣ����ŵ�������Ŀ�Ļ���ȥ��֤��

��ϰ��ϵ�д�
�����Ŀ