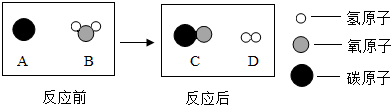
��Ŀ����
����������Ƽ��������ȶ��벻����ѧ��
��ʳƷ����һֱ�����ǹ�ע�Ļ��⣮
��1���ڽ����м�����ǿ���������ҹ�Ϊ���______�����״���״���ƶѪ���������ɡ�����ʵʩ����Ŀ��
��2������ʢ���Ĵ��и�������Ӫ���أ����ࡢ��֬�������ʣ��е�______��
���ϵ�Ӧ���뷢չ����������ǵ���������������ֲ��ϵĶ�Ӧ��ĸ��գ�
A���л��ϳɲ��ϡ������� B���������ϡ���������C����Ȼ�л��߷��Ӳ���
��1��������Ʒ����______����2��������ë����______��
��3����������������ǡ�����ɻ��ȵ��ѺϽ�����______��
���ܡ����š���̼�������ǵ�������������ɣ�
��1������______������Դ����һ�������������Բ��ֽ����ʯ��Դ��ȱ���⣬�����Լ��ٶԻ�������Ⱦ��
��2��úȼ��ʱ�����ʵ������ʣ����Լ���______���壨д��ѧʽ���͵�����������к������ŷš���Ч����������γɣ�
�⣺��1���ڽ����м�����ǿ����������Ϊ���岹����Ԫ�أ��Ӷ���ֹƶѪ����
���ƶѪ��
��2�����и�������Ӫ�����ǵ����ʣ�
��������ʣ�
II����1��������Ʒ�����л��ϳɲ��ϣ�
���A��
��2��������ë������Ȼ�л��߷��Ӳ��ϣ�
���C��
��3���ѺϽ����ڽ������ϣ�
���B��
��1��̫���ܡ����ܡ�ˮ�ܡ����ܡ������ܵȶ���������Դ��
���̫���ܣ�
��2��úȼ��ʱ�ܹ����������Ķ�����������ʣ�����ʯ��ʯ���Լ��ٶ���������ŷţ���������Ļ�ѧʽ��SO2��
���SO2��
��������1������ȱ����Ԫ��ʱ��ƶѪ֢��
��2�������к��зḻ�ĵ����ʣ�
���ϰ����������ϡ����ǽ������ϡ��л��ϳɲ��ϡ����ϲ��ϵȣ�
��1����Դ���ٶ�ȱ��������������Դ������Ҫ���壬̫���ܡ����ܡ�ˮ�ܡ����ܡ������ܵȶ���������Դ��
��2����ʯȼ��ȼ��ʱ���ܹ����������Ķ����������������Ⱦ���������ʣ�
�������������ȱ��ijЩӪ����ʱ�����������Ҫ�������ȡ����ʳ��Ա�֤�Ը���Ӫ���صľ������գ��ٽ����彡����չ��
���ƶѪ��
��2�����и�������Ӫ�����ǵ����ʣ�
��������ʣ�
II����1��������Ʒ�����л��ϳɲ��ϣ�
���A��
��2��������ë������Ȼ�л��߷��Ӳ��ϣ�
���C��
��3���ѺϽ����ڽ������ϣ�
���B��
��1��̫���ܡ����ܡ�ˮ�ܡ����ܡ������ܵȶ���������Դ��
���̫���ܣ�
��2��úȼ��ʱ�ܹ����������Ķ�����������ʣ�����ʯ��ʯ���Լ��ٶ���������ŷţ���������Ļ�ѧʽ��SO2��
���SO2��
��������1������ȱ����Ԫ��ʱ��ƶѪ֢��
��2�������к��зḻ�ĵ����ʣ�
���ϰ����������ϡ����ǽ������ϡ��л��ϳɲ��ϡ����ϲ��ϵȣ�
��1����Դ���ٶ�ȱ��������������Դ������Ҫ���壬̫���ܡ����ܡ�ˮ�ܡ����ܡ������ܵȶ���������Դ��
��2����ʯȼ��ȼ��ʱ���ܹ����������Ķ����������������Ⱦ���������ʣ�
�������������ȱ��ijЩӪ����ʱ�����������Ҫ�������ȡ����ʳ��Ա�֤�Ը���Ӫ���صľ������գ��ٽ����彡����չ��

��ϰ��ϵ�д�
 ������Ӧ�������������ϵ�д�
������Ӧ�������������ϵ�д� �㽭֮�ǿ�ʱ�Ż���ҵϵ�д�
�㽭֮�ǿ�ʱ�Ż���ҵϵ�д�
�����Ŀ
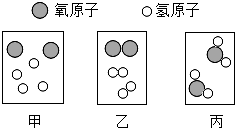 ��2013?����һģ������������Ƽ��������ȶ��벻����ѧ��
��2013?����һģ������������Ƽ��������ȶ��벻����ѧ�� ����ʾһ�������ӣ���
����ʾһ�������ӣ��� ����ʾ
����ʾ