��Ŀ����
�����и�Ԫ����Ҫ�����ڹ����������У����ǻ�����ƾ���[Ca10(PO4)6(OH)2]��ʽ���ڣ�����Է�������Ϊ1004��ţ�̺��Ʒḻ�������գ���ţ���иƺ��ױ������ʣ��ǽ��ǵ�����ʳƷ����ͼ��ij��ҵ��˾��ţ�̰�װ��ǩ�IJ������֡�����ϸ�Ķ���ش��������⣺
��1����װ��ǩ��֬����3.3g����ָ100mLţ���к�֬������������3.3g����ôһ��ţ�̺������� g��������0.01g����
��2�����ǻ�������и�Ԫ�ص���������������Ϊ0.1%����
��3��������ÿ��������Ҫ0.6g�ƣ�����Щ����90%����ţ�̣���һ����ÿ������Ҫ�ȶ��ٺ�ţ�̣�

���𰸡�
��1�� 0.28 g ����1�֣�
��2���⣺����������ԣ�39.8%��1�֣���(��ʽ1��)
��3���⣺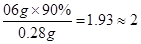
��������
�����������1��һ��ţ�̺���Ԫ�ص�������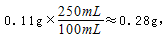 ���ʴ�Ϊ��0.28����2���ǻ�������и�Ԫ�ص�����������
���ʴ�Ϊ��0.28����2���ǻ�������и�Ԫ�ص�����������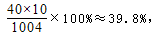 �ʴ�Ϊ��39.8%����3��0.6g��90%��0.28g=2�У���һ����ÿ������Ҫ��2��ţ�̣�
�ʴ�Ϊ��39.8%����3��0.6g��90%��0.28g=2�У���һ����ÿ������Ҫ��2��ţ�̣�
���㣺Ԫ�����������ļ��㣮

��ϰ��ϵ�д�
 ��ĩ���ƾ�ϵ�д�
��ĩ���ƾ�ϵ�д� ���ɿ��ñ���ϵ�д�
���ɿ��ñ���ϵ�д�
�����Ŀ
 �����и�Ԫ����Ҫ�����ڹ����������У����ǻ�����ƾ���[Ca10��PO4��6��OH��2]��ʽ���ڣ�����Է�������Ϊ
�����и�Ԫ����Ҫ�����ڹ����������У����ǻ�����ƾ���[Ca10��PO4��6��OH��2]��ʽ���ڣ�����Է�������Ϊ �����и�Ԫ����Ҫ�����ڹ����������У����ǻ�����ƾ���[Ca10��PO4��6��OH��2]��ʽ���ڣ�����Է�������Ϊ________��ţ�̺��Ʒḻ���������գ���ţ�̺Ƶı������У��ǽ��ǵ�����ʳƷ����ͼ��ij��ҵ��˾��ţ�̰�װ��ǩ��һ�������֣�����ϸ�Ķ���ش����⣺
�����и�Ԫ����Ҫ�����ڹ����������У����ǻ�����ƾ���[Ca10��PO4��6��OH��2]��ʽ���ڣ�����Է�������Ϊ________��ţ�̺��Ʒḻ���������գ���ţ�̺Ƶı������У��ǽ��ǵ�����ʳƷ����ͼ��ij��ҵ��˾��ţ�̰�װ��ǩ��һ�������֣�����ϸ�Ķ���ش����⣺
 �����и�Ԫ����Ҫ�����ڹ����������У����ǻ�����
�����и�Ԫ����Ҫ�����ڹ����������У����ǻ�����