��Ŀ����
ʯ��ʯ�Dz�ƽ����Ҫ���֮һ��С��ͬѧΪ��Ѱ�Һ�̼��Ƴ���82%��ʯ��ʯ���Բɼ���ʯ��ʯ��Ʒ���������¶���ʵ�飮��ͬѧ�Dz����о���
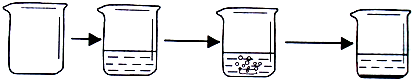
| ʵ�鲽�� | �ٳ�ȡ�ձ��������� | �ڽ�������������ձ��в����أ� | �۳�ȡ����ʯ��ʯ��Ʒ������ʢ��ϡ������ձ��У�ʹ֮��ϡ�����ַ�Ӧ�� | �ܴ���Ӧ��ȫ���أ� |
| ʵ��ͼʾ | 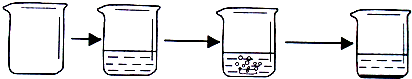 | |||
| ʵ������ | �ձ�������Ϊ40.0g | �ձ������������Ϊ90.0g | ʯ��ʯ��Ʒ������Ϊ12.0g | �ձ������л���������Ϊ97.6g |
�⣺��1�����������غ㶨�ɵã���Ӧ�ų�CO2������=��90.0g+12.0g��-97.6g=4.4g��
��2����12.0gʯ��ʯ��Ʒ�к�CaCO3����Ϊx
CaCO3+2HCl�TCaCl2+CO2��+H2O
100 44
x 4.4g
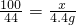
x�T10.0g
��ʯ��ʯ��Ʒ�Ĵ���Ϊ��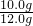 ��100%=83.3%
��100%=83.3%
��83.3%��82%�����Ը�ʯ��ʯ��Ʒ�Ĵ��ȷ���Ҫ��
�𣺣�1����ʵ�������ɵĶ�����̼��������4.4g��
��2����ʯ��ʯ��Ʒ�Ĵ��ȷ���Ҫ��
��������1��ʯ��ʯ����Ҫ�ɷ�̼��ƿ��������ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼�����ŷ�Ӧ�ķ�����������̼���Ϸų���ʹ�ձ�������������С����ȫ��Ӧǰ���ձ�������������Ϊ�ų����������̼�����������Ծݴ����������̼��������
��2�����ݷ�Ӧ�Ļ�ѧ����ʽ�����ɷ�Ӧ�ų�������̼����������μӷ�Ӧ��̼��Ƶ�������Ȼ�����ʯ��ʯ��Ʒ�Ĵ���= ��100%�����̼��ƵĴ��ȣ���82%�Ƚϼ��ɣ�
��100%�����̼��ƵĴ��ȣ���82%�Ƚϼ��ɣ�
���������������غ㶨�ɣ��ų�����ķ�Ӧ����Ӧǰ�����ʵ��������뷴Ӧ��ʣ�����ʵ������Ϊ��Ӧ�ų������������
��2����12.0gʯ��ʯ��Ʒ�к�CaCO3����Ϊx
CaCO3+2HCl�TCaCl2+CO2��+H2O
100 44
x 4.4g
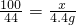
x�T10.0g
��ʯ��ʯ��Ʒ�Ĵ���Ϊ��
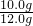 ��100%=83.3%
��100%=83.3%��83.3%��82%�����Ը�ʯ��ʯ��Ʒ�Ĵ��ȷ���Ҫ��
�𣺣�1����ʵ�������ɵĶ�����̼��������4.4g��
��2����ʯ��ʯ��Ʒ�Ĵ��ȷ���Ҫ��
��������1��ʯ��ʯ����Ҫ�ɷ�̼��ƿ��������ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼�����ŷ�Ӧ�ķ�����������̼���Ϸų���ʹ�ձ�������������С����ȫ��Ӧǰ���ձ�������������Ϊ�ų����������̼�����������Ծݴ����������̼��������
��2�����ݷ�Ӧ�Ļ�ѧ����ʽ�����ɷ�Ӧ�ų�������̼����������μӷ�Ӧ��̼��Ƶ�������Ȼ�����ʯ��ʯ��Ʒ�Ĵ���=
 ��100%�����̼��ƵĴ��ȣ���82%�Ƚϼ��ɣ�
��100%�����̼��ƵĴ��ȣ���82%�Ƚϼ��ɣ����������������غ㶨�ɣ��ų�����ķ�Ӧ����Ӧǰ�����ʵ��������뷴Ӧ��ʣ�����ʵ������Ϊ��Ӧ�ų������������

��ϰ��ϵ�д�
 ��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
�����Ŀ
ʯ��ʯ�Dz�ƽ����Ҫ���֮һ��С��ͬѧΪ��Ѱ�Һ�̼��Ƴ���82%��ʯ��ʯ���Բɼ���ʯ��ʯ��Ʒ���������¶���ʵ�飮��ͬѧ�Dz����о���
��ͨ���������㣺��1����ʵ�������ɶ�����̼�������Ƕ��ٿˣ���2����ʯ��ʯ��Ʒ��̼��ƺ����Ƿ����Ҫ������ʯ��ʯ��Ʒ�е����ʲ������ᷴӦҲ������ˮ��
| ʵ�鲽�� | �ٳ�ȡ�ձ��������� | �ڽ�������������ձ��в����أ� | �۳�ȡ����ʯ��ʯ��Ʒ������ʢ��ϡ������ձ��У�ʹ֮��ϡ�����ַ�Ӧ�� | �ܴ���Ӧ��ȫ���أ� |
| ʵ��ͼʾ | 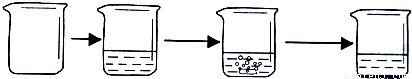 | |||
| ʵ������ | �ձ�������Ϊ40.0g | �ձ������������Ϊ90.0g | ʯ��ʯ��Ʒ������Ϊ12.0g | �ձ������л���������Ϊ97.6g |