��Ŀ����
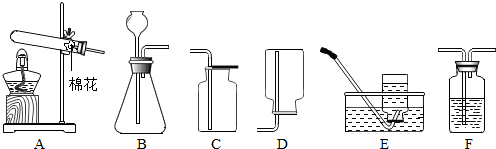
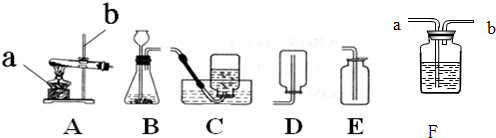
ʵ���ҳ�������װ���Ʊ����壬��ش��������⡣
 |
��1��д���б�����������ƣ���_____ _____����_____ ______��
______��
��2��ʵ����������غͶ���������ȡ��������Ӧ�Ļ�ѧ����ʽΪ
_ _____ ___ ��
Ӧѡ�õ����巢��װ����_______(���ţ���ͬ)��
Ϊ����©��������ҩƷǰ��Ӧ�ȼ��װ�õ�___________��
��3���ô���ʯ��ϡ������ȡ������̼�Ļ�ѧ����ʽΪ ��
��װ��_______�����ţ��ռ�һƿ������̼����ȼ�ŵ�ľ���ӽ�ƿ�ڣ����۲쵽 ��˵��ƿ���ѳ���������̼��
��4���ռ������Ͷ� ����̼����ѡ�õ���ͬװ����________�����ţ�����ԭ����____________
����̼����ѡ�õ���ͬװ����________�����ţ�����ԭ����____________
��1������©�� ����ƿ
MnO2
 ��2��2KClO3=====2KCl+3O2
��2��2KClO3=====2KCl+3O2
Aװ�� ������
��3��CaCO3+2HCl==CaCl2+H2O+CO2
Cװ�� ľ��Ϩ�� Cװ�� ����������ܶȾ����ڿ������ܶ�

��ϰ��ϵ�д�
 ��ʦ�㾦�ִʾ��ƪϵ�д�
��ʦ�㾦�ִʾ��ƪϵ�д�
�����Ŀ