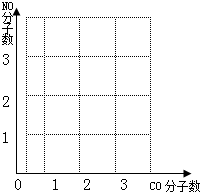
��Ŀ����
�״���CH3OH���ж���������ʹ�۾�ʧ�������������������о�֤���ð�����NH3���������м״��Ĺ�ҵ��ˮ��ʹ��ת����������ʣ��йط�Ӧ�Ļ�ѧ����ʽΪ��5CH3OH+12O2+6NH3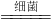 3B+5CO2+19H2O
3B+5CO2+19H2O
��1��������Ӧ��B���ʵĻ�ѧʽΪ______���״�����Ԫ�ص���������Ϊ______��
��2������������0.32%�״��Ĺ�ҵ��ˮ500t����������Ҫ�������ٶ֣�
�⣺��1����Ӧǰԭ�Ӽ�����ͳ�ƽ��Ϊ��C 10��H 38��O 29��N 6��
����Ӧ����֪�����и�ԭ�Ӽ�����ΪC 10��H 38��O 29��
��Ӧǰ����Ƚϣ��ɵã���Ӧ��ȱ��6��Nԭ�ӣ���3B��Ӧ����6��Nԭ�ӣ�����B���ʵĻ�ѧʽΪN2��
�ʴ�N2
���ݼ״��Ļ�ѧʽCH3OH���״�����Ԫ�ص���������=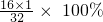 =50%��
=50%��
�ʴ�50%
��2������Ҫ����������Ϊx
5CH3OH+12O2+6NH3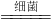 3N2+5CO2+19H2O
3N2+5CO2+19H2O
160 102
500t��0.32% x
160��102=��500t��0.32%����x
��֮�� x=1.02t
����������Ҫ����1.02�֣�
��������1����Ӧ��B���ʵĻ�ѧʽҪ���������غ㶨�ɣ���Ӧǰ��ԭ�����ࡢ�������䣬�����ƶϣ����ݻ�ѧʽ�������Ԫ�ص����������Ļ������㣻
��2�����û�ѧ����ʽ5CH3OH+12O2+6NH3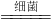 3N2+5CO2+19H2O���ɷ�Ӧ��״����������㷴Ӧ�ﰱ����������
3N2+5CO2+19H2O���ɷ�Ӧ��״����������㷴Ӧ�ﰱ����������
��������ҵ�ƾ��д�Լ����4%�ļ״������������ӵ���ʳ�þƾ������پƣ����������úͻ�����״��ж���
����Ӧ����֪�����и�ԭ�Ӽ�����ΪC 10��H 38��O 29��
��Ӧǰ����Ƚϣ��ɵã���Ӧ��ȱ��6��Nԭ�ӣ���3B��Ӧ����6��Nԭ�ӣ�����B���ʵĻ�ѧʽΪN2��
�ʴ�N2
���ݼ״��Ļ�ѧʽCH3OH���״�����Ԫ�ص���������=
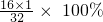 =50%��
=50%���ʴ�50%
��2������Ҫ����������Ϊx
5CH3OH+12O2+6NH3
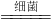 3N2+5CO2+19H2O
3N2+5CO2+19H2O160 102
500t��0.32% x
160��102=��500t��0.32%����x
��֮�� x=1.02t
����������Ҫ����1.02�֣�
��������1����Ӧ��B���ʵĻ�ѧʽҪ���������غ㶨�ɣ���Ӧǰ��ԭ�����ࡢ�������䣬�����ƶϣ����ݻ�ѧʽ�������Ԫ�ص����������Ļ������㣻
��2�����û�ѧ����ʽ5CH3OH+12O2+6NH3
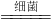 3N2+5CO2+19H2O���ɷ�Ӧ��״����������㷴Ӧ�ﰱ����������
3N2+5CO2+19H2O���ɷ�Ӧ��״����������㷴Ӧ�ﰱ������������������ҵ�ƾ��д�Լ����4%�ļ״������������ӵ���ʳ�þƾ������پƣ����������úͻ�����״��ж���

��ϰ��ϵ�д�
�����Ŀ

 ��κ�������Ч����Լ��ʹ��ú��ʯ�͡���Ȼ����������������Ҫ�Ļ�ʯȼ�ϣ��ӽ��������������������Դ��ȫ�������ٵĹ�ͬ���⣬�Ƿ�չ��̼���õĵ���֮�����ش��������⣺
��κ�������Ч����Լ��ʹ��ú��ʯ�͡���Ȼ����������������Ҫ�Ļ�ʯȼ�ϣ��ӽ��������������������Դ��ȫ�������ٵĹ�ͬ���⣬�Ƿ�չ��̼���õĵ���֮�����ش��������⣺