��Ŀ����
��ͼ��ʾ���ô�װ�ò��������������ĺ�������ȼ�ճ��м�����ף�����������е�����������ѧ��Ӧ���������������壬�밴Ҫ����գ�
��1��д��������������ƣ���______��______��
��2���ڿ����е�ȼ���еĺ��ף���Ѹ�ٲ��벣�����ڣ�������Ƥ������ʱ��������Ҫ������______���ñ仯�����ֱ���ʽΪ______��
��3��������ȼ����ϣ�������ȴ�����º���������ˮ��ı仯��______�����ָ������ԭ���ǣ�����ȼ�������˿�������̬��______�������˹�̬��______���Ӷ�ʹ��������������______ ����������С�����䡱��������������ѹǿ______ �������ڡ�����С�ڡ����ڡ���������Ĵ���ѹ��
��4������ʵ����Կ��Եó�������Լռ�������______���Ľ����⣬�����Եó��йص��������ʽ�����______��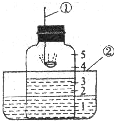
��1��д��������������ƣ���______��______��
��2���ڿ����е�ȼ���еĺ��ף���Ѹ�ٲ��벣�����ڣ�������Ƥ������ʱ��������Ҫ������______���ñ仯�����ֱ���ʽΪ______��
��3��������ȼ����ϣ�������ȴ�����º���������ˮ��ı仯��______�����ָ������ԭ���ǣ�����ȼ�������˿�������̬��______�������˹�̬��______���Ӷ�ʹ��������������______ ����������С�����䡱��������������ѹǿ______ �������ڡ�����С�ڡ����ڡ���������Ĵ���ѹ��
��4������ʵ����Կ��Եó�������Լռ�������______���Ľ����⣬�����Եó��йص��������ʽ�����______��

��1����ȼ�ճ� ��ˮ��
��2������ȼ���������������ף���Ӧ�������ǣ�����ȼ�գ����ƹ⡢���ȡ����������İ��̣��ʴ�Ϊ������ȼ�գ����ƹ⡢���ȡ����������İ��̣�����+����
����������
��3�������ں���ȼ�����ĵ�����������������ѹǿ��С����֪����Լռ�������
������ѹ�Ͱ�ˮѹ�˲�����ٵ��������������Լ
�����������O2�����������ף���С��С��
��4��ʵ���е�װ���ڽ���ˮ�Ժ�����Ȼ�ܹ��������˵�������Dz�����ˮ��Ҳ����ˮ��Ӧ�������ں���������Ӧ�Ĺ����У�����û�б����ģ����Ե�����������������Ӧ�����Ը���Ϊ�����ȶ�����ȻҲ���Էֿ����������Dz���������Ӧ����֧��ȼ�յȣ����
�����������ã����������Ӧ������������ˮ
��2������ȼ���������������ף���Ӧ�������ǣ�����ȼ�գ����ƹ⡢���ȡ����������İ��̣��ʴ�Ϊ������ȼ�գ����ƹ⡢���ȡ����������İ��̣�����+����
| ||
��3�������ں���ȼ�����ĵ�����������������ѹǿ��С����֪����Լռ�������
| 1 |
| 5 |
| 1 |
| 5 |
��4��ʵ���е�װ���ڽ���ˮ�Ժ�����Ȼ�ܹ��������˵�������Dz�����ˮ��Ҳ����ˮ��Ӧ�������ں���������Ӧ�Ĺ����У�����û�б����ģ����Ե�����������������Ӧ�����Ը���Ϊ�����ȶ�����ȻҲ���Էֿ����������Dz���������Ӧ����֧��ȼ�յȣ����
| 1 |
| 5 |

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ

 ��ͼ��ʾ���ô���װ�ò��������������ĺ�������ȼ�ճ��м�����ף�����������е�����������ѧ��Ӧ���������������壬�밴Ҫ����գ�
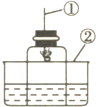
��ͼ��ʾ���ô���װ�ò��������������ĺ�������ȼ�ճ��м�����ף�����������е�����������ѧ��Ӧ���������������壬�밴Ҫ����գ�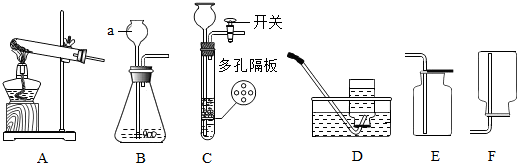
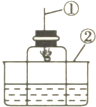 ��ͼ��ʾ���ô���װ�ò��������������ĺ�������ȼ�ճ��м�����ף�����������е�����������ѧ��Ӧ���������������壬�밴Ҫ����գ�
��ͼ��ʾ���ô���װ�ò��������������ĺ�������ȼ�ճ��м�����ף�����������е�����������ѧ��Ӧ���������������壬�밴Ҫ����գ�