��Ŀ����
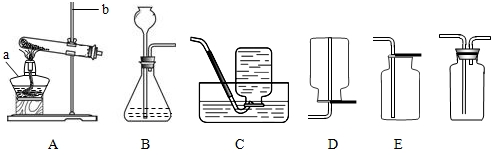
(1)(6��)��������������壬������ˮ��������Ϊδ����������Դ���Ǻ��칤ҵ�ĸ���ȼ�ϣ�Ҳ�ǻ�����������Ҫԭ�ϡ�ʵ����ͨ����������װ����ȡ���������������Ҫ��ش�
Ҫ��ش�
��ʵ����ʹ��ϡ����ͽ���п��ȡ�����Ļ�ѧ��Ӧ����ʽΪ__________________�����ַ����Ƶõ�����������������________________(ԭ����__________________________)��ˮ�������ɽ���������ͨ��װ��_________________��ȥ��
��ʵ���пɲ���__________װ���ռ�����������������ļ���ƿ��װ��G��ʽ���ñ��ã�ԭ����__________________________________��
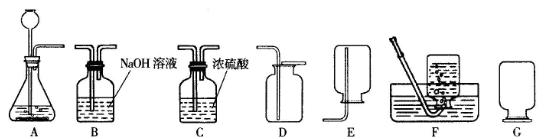
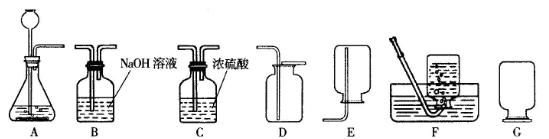
(2)(5��)ijУ�����о�С�鵽��Ƴ����죬�˽������ͭ����Ҫ�����������£�

(2)(5��)ijУ�����о�С�鵽��Ƴ����죬�˽������ͭ����Ҫ�����������£�
����������ṩ����Ϣ�ش�
�����̢��ˮ�к��е�������_________________________________��
�ھ��ⶨ�����̢��з�ˮ��pHΪ4����ˮ��һ��û��________________________��
�����̢���ˮ�г����̢�����������⣬������_______________________��
����Ƽ�����֤������ƶϣ�
______________________________________________________________________________��
(1)��Zn+2HCl=ZnCl2+H2�� �Ȼ��� �����лӷ���B��c
��E��F �����ȿ����ᣬ�Է��ݳ�
(2)��H2S04��Fe2(S04)3��FeS04 ��NaOH
��CuSO4
ȡ������ˮ������һ��ĥ��������Ƭ��һ��ʱ���ȡ���۲죬��Ƭ���к�ɫ��ͭ��˵
����ˮ�к���CuS04
˵����(1)��(2)С���ȫ��ȷ�ɵ÷֣����ƺͻ�ѧʽͬ���÷�
���ֱ�����(2)����Ŀ�2�֣�����ÿ��l�֣���11�ֽ���:
��
��E��F �����ȿ����ᣬ�Է��ݳ�
(2)��H2S04��Fe2(S04)3��FeS04 ��NaOH
��CuSO4
ȡ������ˮ������һ��ĥ��������Ƭ��һ��ʱ���ȡ���۲죬��Ƭ���к�ɫ��ͭ��˵
����ˮ�к���CuS04
˵����(1)��(2)С���ȫ��ȷ�ɵ÷֣����ƺͻ�ѧʽͬ���÷�
���ֱ�����(2)����Ŀ�2�֣�����ÿ��l�֣���11�ֽ���:
��

��ϰ��ϵ�д�
 ����˼ά����ѵ����ʱ��ѧ��ϵ�д�
����˼ά����ѵ����ʱ��ѧ��ϵ�д�
�����Ŀ