��Ŀ����
����������һ����Ҫ�Ļ�������ԭ�ϣ��㷺��Ӧ������ֽ����֯��ʯ�ͻ�����ӡȾ����ҵ��
��1���������Ƶ�������______��ֻдһ�֣�����������������ͭ��Ӧ�Ļ�ѧ����ʽΪ______��
��2��ʵ������һƿ���õĹ��壬��ǩ��д�š��������ơ���Ϊ�˼��������Ƿ���NaOH��ʵ��Աȡ�����ù�����Ʒ��������ʵ�飺
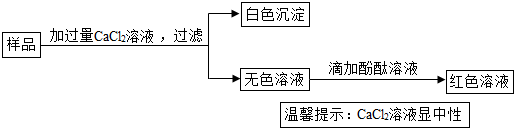
������Һ�����һ����˵����Ʒ�к���______������ţ���
A��CaCl2�� B��Na2CO3����C��NaOH�� D��Na2CO3��NaOH��
�⣺��1�����������׳�Ϊ���ռ�������Ƶȣ���������������ͭ��Ӧ���������ƺ�������ͭ���ù۲취��ƽ��������ͭ������ϳ������ţ����Է�Ӧ�Ļ�ѧ����ʽΪ��2NaOH+CuSO4�TNa2SO4+Cu��OH��2����
��2���μӷ�̪��Һ��Һ��죬˵����Һ�Լ��ԣ��ʺ����������ƣ�̼������ȻҲ�Լ��ԣ������ڼ����˹������Ȼ��ƣ�������Һ����̼���ƣ���ѡC��
�ʴ�Ϊ����1���� 2NaOH+CuSO4=Na2SO4+Cu��OH��2������2��C��
��������1�������������Ƶ��׳�Ϊ�ռ�������ƽ��з�����������������������ͭ��Ӧ���������ƺ�������ͭ��ȷ��д��ѧ����ʽ��
��2���������������Լ��Կ�ʹ��̪��Һ�����з����ش𣻸���̼��Ʋ�����ˮ�����������ܽ��з����ش�
���������⿼���˳������ʵ��׳Ƽ����ʳɷֵ�̽���ͻ�ѧʽ�ͻ�ѧ����ʽ����д����ɴ��⣬�����������ʵ����ʽ��У�
��2���μӷ�̪��Һ��Һ��죬˵����Һ�Լ��ԣ��ʺ����������ƣ�̼������ȻҲ�Լ��ԣ������ڼ����˹������Ȼ��ƣ�������Һ����̼���ƣ���ѡC��
�ʴ�Ϊ����1���� 2NaOH+CuSO4=Na2SO4+Cu��OH��2������2��C��
��������1�������������Ƶ��׳�Ϊ�ռ�������ƽ��з�����������������������ͭ��Ӧ���������ƺ�������ͭ��ȷ��д��ѧ����ʽ��
��2���������������Լ��Կ�ʹ��̪��Һ�����з����ش𣻸���̼��Ʋ�����ˮ�����������ܽ��з����ش�
���������⿼���˳������ʵ��׳Ƽ����ʳɷֵ�̽���ͻ�ѧʽ�ͻ�ѧ����ʽ����д����ɴ��⣬�����������ʵ����ʽ��У�

��ϰ��ϵ�д�
�����Ŀ