��Ŀ����
Ϊ���о�CO2�����ʣ���Ҫ��ȡ���ռ������CO2���壮��������ʦ�ṩ��һЩʵ��װ�ã�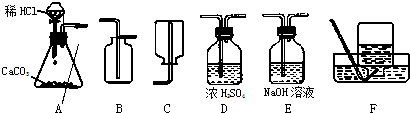
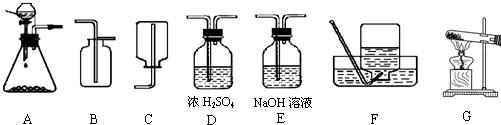
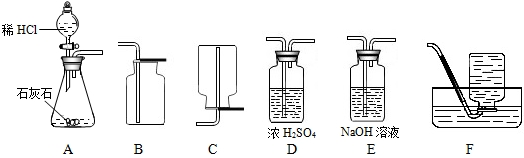
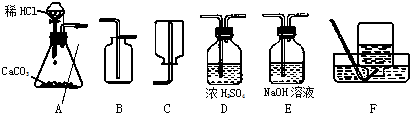
��1����ȡ���ռ������CO2���壬�ɲ��õ�װ�������________������ĸ����
��2��ʵ������ȡCO2�Ļ�ѧ����ʽΪ________��
��3����������ʵ�����������ռ���������ܵ�ԭ����________��
��4��ʵ������ȡCO2��________����ܡ����ܡ�����ϡHCl����ŨHCl�������ǣ�________��
��5����ȡ11gʯ��ʯ���������ᷴӦ������4.4g������̼����������ʧ�������ʯ��ʯ��̼��Ƶ����������Ƕ��٣���C-12��O-16��Ca-40��
�⣺��1��ʵ�����ô���ʯ��ϡ���ᷴӦ��ȡ������̼����Ӧ���״�ǹ����Һ�壬��Ӧ�����dz��£�Ӧѡ�÷���װ��A�����ڶ�����̼���ܶȴ��ڿ������ܶȣ���ѡ���ռ�װ��B�����ռ�ǰ��Ũ������и�����Կɲ��õ�װ�������ADB��
��2��ʵ������ȡCO2�Ļ�ѧ����ʽΪ��CaCO3 +2HCl�TCaCl2+H2O+CO2����
��3����������ʵ�����������ռ���������ܵ�ԭ����ҩƷ���㡢װ��©���ȣ�
��4��ʵ������ȡCO2�����ܽ�ϡHCl����ŨHCl�������ǣ���ΪŨ�����ӷ���������HCl����ʹ�Ƴ������岻����
��5����ʯ��ʯ��̼��Ƶ�����Ϊx��
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
X 4.4g
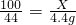 ��ã�X=10g
��ã�X=10g
ʯ��ʯ��̼��Ƶ�����������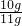 ��100%��90.9%
��100%��90.9%
�ʴ�Ϊ����1��ADB�� ��2��CaCO3 +2HCl�TCaCl2+H2O+CO2���� ��3��ҩƷ���㡢װ��©���ȣ���4��������ΪŨ�����ӷ���������HCl����ʹ�Ƴ������岻������5��ʯ��ʯ��̼�������������90.9%��
��������1������ʵ������ȡ������̼�ķ�Ӧ���״̬����Ӧ����ȷ������װ�ã����ݶ�����̼���ռ�����ȷ���ռ�װ�ã���ȡ�Ķ�����̼����Ũ������и��
��2������ʵ������ȡ������̼�ķ�Ӧ��д����Ӧ�ķ���ʽ��
��3��������ȡ������̼�IJ��輰ע�����������
��4������Ũ����Ļӷ��Խ��з�����
��5������̼��������ᷴӦ�ķ���ʽ�ɶ�����̼���������̼��Ƶ������������ʯ��ʯ��̼��Ƶ�����������
����������Ϊʵ������ȡ������̼��һ���ۺ��⣬���������̼��ʵ�����Ʒ�ԭ������ȡװ�á��ռ�װ�õ�ѡ���Լ�������̼��ijЩ���ʣ�NaOH��Һ�ܺͶ�����̼��Ӧ��
��2��ʵ������ȡCO2�Ļ�ѧ����ʽΪ��CaCO3 +2HCl�TCaCl2+H2O+CO2����
��3����������ʵ�����������ռ���������ܵ�ԭ����ҩƷ���㡢װ��©���ȣ�
��4��ʵ������ȡCO2�����ܽ�ϡHCl����ŨHCl�������ǣ���ΪŨ�����ӷ���������HCl����ʹ�Ƴ������岻����
��5����ʯ��ʯ��̼��Ƶ�����Ϊx��
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
X 4.4g
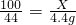 ��ã�X=10g
��ã�X=10gʯ��ʯ��̼��Ƶ�����������
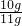 ��100%��90.9%
��100%��90.9%�ʴ�Ϊ����1��ADB�� ��2��CaCO3 +2HCl�TCaCl2+H2O+CO2���� ��3��ҩƷ���㡢װ��©���ȣ���4��������ΪŨ�����ӷ���������HCl����ʹ�Ƴ������岻������5��ʯ��ʯ��̼�������������90.9%��
��������1������ʵ������ȡ������̼�ķ�Ӧ���״̬����Ӧ����ȷ������װ�ã����ݶ�����̼���ռ�����ȷ���ռ�װ�ã���ȡ�Ķ�����̼����Ũ������и��
��2������ʵ������ȡ������̼�ķ�Ӧ��д����Ӧ�ķ���ʽ��
��3��������ȡ������̼�IJ��輰ע�����������
��4������Ũ����Ļӷ��Խ��з�����
��5������̼��������ᷴӦ�ķ���ʽ�ɶ�����̼���������̼��Ƶ������������ʯ��ʯ��̼��Ƶ�����������
����������Ϊʵ������ȡ������̼��һ���ۺ��⣬���������̼��ʵ�����Ʒ�ԭ������ȡװ�á��ռ�װ�õ�ѡ���Լ�������̼��ijЩ���ʣ�NaOH��Һ�ܺͶ�����̼��Ӧ��

��ϰ��ϵ�д�
�����Ŀ