��Ŀ����
�Ե��ʳ��ˮΪ������ȡ�������������ƵȲ�Ʒ�Ĺ�ҵ��Ϊ���ȼҵ��������Ŀǰ��ѧ��ҵ����Ҫ֧��֮һ�����ڴ����к�������MgCl2��CaCl2��Na2SO4�����ʣ������ϵ��Ҫ����˱��뾭�����ơ��Դ���Ϊԭ�ϵġ��ȼҵ���������£�
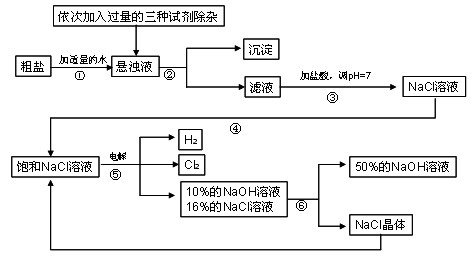
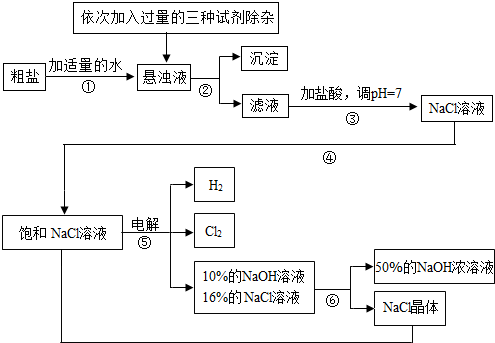
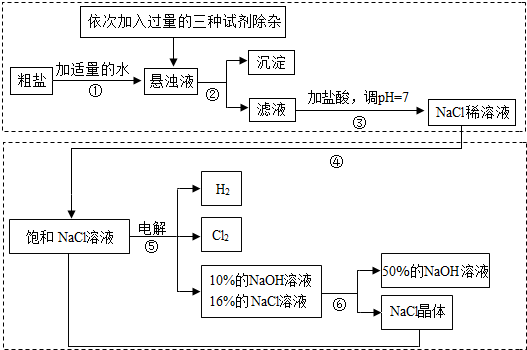
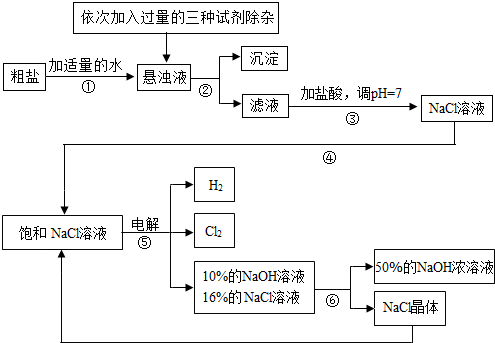
�ش��������⣺
��1�����������������ڹ�ҵ���й㷺����;�����й����������Ƶ������У��������________��
A����ȥ�����ۣ������������� B.������ˮ���ܽ�ʱ�ų��������� C��ˮ��Һ��ʹʯ����Һ��� D.������ijЩ����ĸ����
��2�������ڵ�������______�������ܵ�������________��
��3�������٢ڼ������ʱ���ӵ������Լ���NaOH��Һ��Na2CO3��Һ��BaCl2��Һ��������������˳��Ҫ���ǣ�Na2CO3��Һ������BaCl2��Һ֮________���ǰ�������롣��ͬѧ�����_____��Һ����BaCl2��Һ�ɴﵽͬ����Ŀ�ġ�
��4����ⱥ��NaCl��Һ�Ļ�ѧ����ʽ��_________________��
��5���������п���ѭ�����õ�������________��
��1�����������������ڹ�ҵ���й㷺����;�����й����������Ƶ������У��������________��
A����ȥ�����ۣ������������� B.������ˮ���ܽ�ʱ�ų��������� C��ˮ��Һ��ʹʯ����Һ��� D.������ijЩ����ĸ����
��2�������ڵ�������______�������ܵ�������________��
��3�������٢ڼ������ʱ���ӵ������Լ���NaOH��Һ��Na2CO3��Һ��BaCl2��Һ��������������˳��Ҫ���ǣ�Na2CO3��Һ������BaCl2��Һ֮________���ǰ�������롣��ͬѧ�����_____��Һ����BaCl2��Һ�ɴﵽͬ����Ŀ�ġ�
��4����ⱥ��NaCl��Һ�Ļ�ѧ����ʽ��_________________��
��5���������п���ѭ�����õ�������________��
��1��C
��2�����ˣ�����
��3����Ba(OH)2
��4��2NaCl+2H2O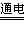 2NaOH+H2��+Cl2��
2NaOH+H2��+Cl2��
��5��NaCl
��2�����ˣ�����
��3����Ba(OH)2
��4��2NaCl+2H2O
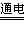 2NaOH+H2��+Cl2��
2NaOH+H2��+Cl2����5��NaCl

��ϰ��ϵ�д�
�����Ŀ