��Ŀ����
�����������ᷢչ�벻����ѧ��
�� ����������벻�������������к������������� ��1�� ���ѧʽ�����ڿ����еĺ�����������������ЧӦ�������� ��2�� ���� ��ѧʽ�������������������������Ҫ���ʣ�����������������Һ���ն�������Ļ�ѧ����ʽ�� ��3�� ��
�� ���������벻��ˮ����ˮ�ľ��������м��������������� ��4�� �����������á�����̿+����Ĥ+�����ߡ���Ϲ��ջ��ֱ��ˮ�����л���̿�� ��5�� ���ã�ҽ���г��õ�������ˮ��0.9%��ʳ��ˮ���ò�˿պȡ�������ڻ��������գ��ɹ۲쵽����� ��6�� ɫ��
�� ��ũ�ҷ���ȣ�������Ч�ɷֶ࣬��Ч�죬����ʹ�û��ʿ�һ���̶��ϴٽ���ʳ��ũ��Ʒ������������һ�ֳ��õĵ��ʣ���ѧʽ��CO(NH2)2������ ��7�� ��Ԫ����ɣ�����NԪ�ص����������� ��8�� ����ȷ��0.1%����0.5mol������Լ���� ��9����Nԭ�ӡ�
�� ����������벻�������������к������������� ��1�� ���ѧʽ�����ڿ����еĺ�����������������ЧӦ�������� ��2�� ���� ��ѧʽ�������������������������Ҫ���ʣ�����������������Һ���ն�������Ļ�ѧ����ʽ�� ��3�� ��
�� ���������벻��ˮ����ˮ�ľ��������м��������������� ��4�� �����������á�����̿+����Ĥ+�����ߡ���Ϲ��ջ��ֱ��ˮ�����л���̿�� ��5�� ���ã�ҽ���г��õ�������ˮ��0.9%��ʳ��ˮ���ò�˿պȡ�������ڻ��������գ��ɹ۲쵽����� ��6�� ɫ��
�� ��ũ�ҷ���ȣ�������Ч�ɷֶ࣬��Ч�죬����ʹ�û��ʿ�һ���̶��ϴٽ���ʳ��ũ��Ʒ������������һ�ֳ��õĵ��ʣ���ѧʽ��CO(NH2)2������ ��7�� ��Ԫ����ɣ�����NԪ�ص����������� ��8�� ����ȷ��0.1%����0.5mol������Լ���� ��9����Nԭ�ӡ�
�٣�1��N2����2��CO2����3��SO2 + 2NaOH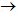 Na2SO3 + H2O��
Na2SO3 + H2O��
�ڣ�4��ɱ����������5����������6���ƣ�
�ۣ�7���ģ���8��46.7%����9��6.02��1023
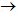 Na2SO3 + H2O��
Na2SO3 + H2O���ڣ�4��ɱ����������5����������6���ƣ�
�ۣ�7���ģ���8��46.7%����9��6.02��1023

��ϰ��ϵ�д�
 ��ѧ�����ϵ�д�
��ѧ�����ϵ�д� �·Ƿ��̸����100��ϵ�д�
�·Ƿ��̸����100��ϵ�д�
�����Ŀ