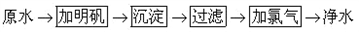��Ŀ����
�ҹ��Ϸ������õ�ˮ����Ϊ��ˮ�����ں�ˮ�к��д�������ɳ���������ϸ�������ʣ����ô����ᣨHClO��ɱ������Ư��[��Ч�ɷ�ΪCa��ClO��2]����ˮ���ڿ�����CO2�����¿�����̼��ƺʹ����ᣮ������ij����������ˮ֮ǰ�Ժ�ˮ�Ĵ������裺
��ˮ �������� ���� ���� ����Ư�� ��ˮ
��1������������������
��2������ˮ��������������
��3���������ƣ�Na2FeO4����һ�ֳ��õ���������Ŀǰ���㷺Ӧ��������ˮ������������������Ԫ�صĻ��ϼ�Ϊ
��4���þ���õ��ľ�ˮ�Ǵ����ﻹ�ǻ�����
��5����ס�����ҵ�С���������ȼ٣����˲��þе��������ʣ�ҽ�����С����������Ϊ��ˮ����������ԭ���Ϸ���ˮӲ�Ƚ�С��������ˮӲ�Ƚϴ�С����������һʱ������Ӧ���������ҽ�����㽫����ˮ�����С��ʲô���飿��
��6��ˮ��Ӳ�ȹ����Ӱ�����������������ˮ��Ӳˮ���õ�������
��ˮ �������� ���� ���� ����Ư�� ��ˮ
��1������������������
����ˮ�������Ĺ���С�����γɴ����
����ˮ�������Ĺ���С�����γɴ����
����2������ˮ��������������
����
����
�ķ�����ȥˮ�в��������ʣ��ٽ���������X��һ�ֳ���������ˮ����������ҵ��ȡX�Ļ�ѧ����ʽΪ��Cl2+2NaClO2=2NaCl+2X����X�Ļ�ѧʽ��ClO2
ClO2
����3���������ƣ�Na2FeO4����һ�ֳ��õ���������Ŀǰ���㷺Ӧ��������ˮ������������������Ԫ�صĻ��ϼ�Ϊ
+6
+6
����4���þ���õ��ľ�ˮ�Ǵ����ﻹ�ǻ�����
�����
�����
���жϵ�������ˮ���ܽ���һЩ����������
ˮ���ܽ���һЩ����������
����5����ס�����ҵ�С���������ȼ٣����˲��þе��������ʣ�ҽ�����С����������Ϊ��ˮ����������ԭ���Ϸ���ˮӲ�Ƚ�С��������ˮӲ�Ƚϴ�С����������һʱ������Ӧ���������ҽ�����㽫����ˮ�����С��ʲô���飿��
����ˮ���ô���ˮ������ˮ
����ˮ���ô���ˮ������ˮ
����6��ˮ��Ӳ�ȹ����Ӱ�����������������ˮ��Ӳˮ���õ�������
����ˮ
����ˮ
����������1�����ݼ�������������������ˮ�������Ĺ���С�����γɴ�������н��
��2�����ݹ��˳�ȥ�����������Լ���ѧ��Ӧǰ��ԭ�ӵ��������Ŀ������н��
��3�����ݻ������и�Ԫ�صĻ��ϼ۵Ĵ�����Ϊ0���н��
��4�����ݾ������Ͼ������п��������ʣ��������ڻ������н��
��5������Ӳˮ�����彡����������������ˮ���潫����ˮ���ô���ˮ������ˮ���н��
��6��������ˮ��Ӳˮ���õ������Ƿ���ˮ��
��2�����ݹ��˳�ȥ�����������Լ���ѧ��Ӧǰ��ԭ�ӵ��������Ŀ������н��
��3�����ݻ������и�Ԫ�صĻ��ϼ۵Ĵ�����Ϊ0���н��
��4�����ݾ������Ͼ������п��������ʣ��������ڻ������н��
��5������Ӳˮ�����彡����������������ˮ���潫����ˮ���ô���ˮ������ˮ���н��
��6��������ˮ��Ӳˮ���õ������Ƿ���ˮ��
����⣺��1����������������������ˮ�������Ĺ���С�����γɴ������
��2�����ݹ��˳�ȥ���������ʣ���ѧ��Ӧǰ��ԭ�ӵ��������Ŀ�����֪��Cl2+2NaClO2=2NaCl+2X����X�Ļ�ѧʽ��ClO2��
��3�����ݻ������и�Ԫ�صĻ��ϼ۵Ĵ�����Ϊ0��֪��������������Ԫ�صĻ��ϼ�Ϊ+6��
��4���������Ͼ������п��������ʣ��������ڻ���
��5��Ӳˮ�����彡����������������ˮ���潫����ˮ���ô���ˮ������ˮ��
��6��������ˮ��Ӳˮ���õ������Ƿ���ˮ�����в�����ĭ�϶������ˮ����ĭ���ٵ���Ӳˮ��
�ʴ�Ϊ����1������ˮ�������Ĺ���С�����γɴ������
��2�����ˣ�ClO2��
��3��+6��
��4������ˮ���ܽ���һЩ���������ʣ�
��5������ˮ���ô���ˮ������ˮ��
��6������ˮ��
��2�����ݹ��˳�ȥ���������ʣ���ѧ��Ӧǰ��ԭ�ӵ��������Ŀ�����֪��Cl2+2NaClO2=2NaCl+2X����X�Ļ�ѧʽ��ClO2��
��3�����ݻ������и�Ԫ�صĻ��ϼ۵Ĵ�����Ϊ0��֪��������������Ԫ�صĻ��ϼ�Ϊ+6��
��4���������Ͼ������п��������ʣ��������ڻ���
��5��Ӳˮ�����彡����������������ˮ���潫����ˮ���ô���ˮ������ˮ��
��6��������ˮ��Ӳˮ���õ������Ƿ���ˮ�����в�����ĭ�϶������ˮ����ĭ���ٵ���Ӳˮ��
�ʴ�Ϊ����1������ˮ�������Ĺ���С�����γɴ������
��2�����ˣ�ClO2��
��3��+6��
��4������ˮ���ܽ���һЩ���������ʣ�
��5������ˮ���ô���ˮ������ˮ��
��6������ˮ��
���������⿼���˾���ˮ��֪ʶ����ɴ��⣬�����������е�֪ʶ���У�

��ϰ��ϵ�д�
 �����ҵ���������ͯ������ϵ�д�
�����ҵ���������ͯ������ϵ�д�
�����Ŀ