��Ŀ����
��7�֣�Ϊ����֤�����غ㶨�ɣ�������С�Թ��зֱ�ע��20g4.9%ϡ�����20g�Ȼ�����Һ������һ�����ձ��г�������ͼ��ʾ
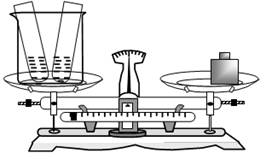
�Ը���Ҫ�ش��������⣺
��1������ֻС�Թ���ҩƷ��ֻ�Ϻ�д���۲쵽��һ��ʵ������ ��
��2����Ϻ�Ӧ����������װ�õ�������֮ǰ��˽�_______����д���и������ţ��� ��û�б仯 ���б仯������������С ���б仯������������� �ܲ���ȷ��
��3����ϡ������ȫ��Ӧ����ͨ�����㣬���ʱ���ò�������Һ����������д����Ӧ������̣���������ȷ��0.01g��
��1���а�ɫ��������
��2����
��3��37.67g
����������1��������Ȼ����������ֽⷴӦ����������������ᱵ��ɫ��������ʵ������Ϊ�а�ɫ�������ɣ�
��2�����������غ㶨�ɿ�֪����ѧ��Ӧǰ�����ʵ����������ֲ��䣬��ѡ�� ��
��3��20g��4.9%=0.98g
�����ɳ���������Ϊx
H2SO4+BaCl2====BaSO4��+2HCl
98 208 233 73
0.98g x
98/0.98g=233/x
x=2.33g
��Һ������Ϊ��20g+20g-2.33g=37.67g
��������Һ������Ϊ37.67g��



 ��2012?��������ģ��Ϊ����֤�����غ㶨�ɣ�������С�Թ��зֱ�ע��20g 4.9%ϡ�����20g�Ȼ�����Һ������һ�����ձ��г�������ͼ��ʾ���Ը���Ҫ�ش��������⣺
��2012?��������ģ��Ϊ����֤�����غ㶨�ɣ�������С�Թ��зֱ�ע��20g 4.9%ϡ�����20g�Ȼ�����Һ������һ�����ձ��г�������ͼ��ʾ���Ը���Ҫ�ش��������⣺