题目内容
某同学为探究铁合金中铁的质量分数,先后进行了四次实验(杂质不与稀硫酸反应),实验数据如下表:根据该同学的实验,试回答以下问题:
| 第一次 | 第二次 | 第三次 | 第四次 | |
| 所取合金的质量∕g | 10 | 10 | 20 | 30 |
| 所加稀硫酸的质量∕g | 100 | 120 | 80 | X |
| 生成氢气的质量∕g | 0.2 | 0.2 | 0.2 | Y |
(2)该铜铁合金中铁的质量分数是多少?
(3)所加稀硫酸溶质质量分数为多少?(结果保留至0.1%).
解:(1)10g:30g=80g:X,解得X=240g;
10g:30g=0.2g:Y,解得Y=0.6g.
(2)设铁合金中铁的质量分数为x.
Fe+H2SO4=FeSO4+H2↑
56 2
30g×x 0.6g
 =
=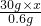 ,x=56%
,x=56%
(3)设稀硫酸中溶质质量分数为y.
Fe+H2SO4=FeSO4+H2↑
56 98
30g×56% 240g×y
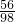 =
= ,y≈12.3%
,y≈12.3%
答:(1)X=240g,Y=0.6g;
(2)铁合金中铁的质量分数为56%;
(3)稀硫酸中溶质质量分数为12.3%.
分析:(1)一、二次实验相比说明10g合金与足量硫酸反应最多生成0.2g氢气;一、三次实验相比说明80g稀硫酸与足量合金反应最多生成0.2g氢气.即10g合金与80g稀硫酸恰好完全反应生成0.2g氢气.由此可以得到X、Y的值.
(2)由第四次实验数据根据化学方程式可以计算出合金中铁的质量分数.
(3)由第四次实验数据根据化学方程式还可以计算出稀硫酸的溶质质量分数.
点评:本题主要考查有关化学方程式的计算和溶质质量分数的计算,难度较大.
10g:30g=0.2g:Y,解得Y=0.6g.
(2)设铁合金中铁的质量分数为x.
Fe+H2SO4=FeSO4+H2↑
56 2
30g×x 0.6g
 =
=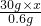 ,x=56%
,x=56%(3)设稀硫酸中溶质质量分数为y.
Fe+H2SO4=FeSO4+H2↑
56 98
30g×56% 240g×y
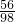 =
= ,y≈12.3%
,y≈12.3%答:(1)X=240g,Y=0.6g;
(2)铁合金中铁的质量分数为56%;
(3)稀硫酸中溶质质量分数为12.3%.
分析:(1)一、二次实验相比说明10g合金与足量硫酸反应最多生成0.2g氢气;一、三次实验相比说明80g稀硫酸与足量合金反应最多生成0.2g氢气.即10g合金与80g稀硫酸恰好完全反应生成0.2g氢气.由此可以得到X、Y的值.
(2)由第四次实验数据根据化学方程式可以计算出合金中铁的质量分数.
(3)由第四次实验数据根据化学方程式还可以计算出稀硫酸的溶质质量分数.
点评:本题主要考查有关化学方程式的计算和溶质质量分数的计算,难度较大.

练习册系列答案
相关题目
某同学为探究铁合金中铁的质量分数,先后进行了三次实验,实验数据如下表:
根据该同学的实验,试回答以下问题:
(1)上表三次实验中,合金里的铁恰好完全反应时,消耗稀硫酸溶液的质量是 g.
(2)该铜铁合金中铁的质量分数是多少? ;
(3)第三次实验所得溶液溶质质量分数为 (结果保留至0.1%).
| 实验次数 项目 |
第一次 | 第二次 | 第三次 |
| 所取合金的质量/g | 20 | 20 | 40 |
| 所加稀硫酸的质量/g | 100 | 120 | 80 |
| 生成氢气的质量/g | 0.4 | 0.4 | 0.4 |
(1)上表三次实验中,合金里的铁恰好完全反应时,消耗稀硫酸溶液的质量是
(2)该铜铁合金中铁的质量分数是多少?
(3)第三次实验所得溶液溶质质量分数为
某同学为探究铁合金中铁的质量分数,先后进行了四次实验(杂质不与稀硫酸反应),实验数据如下表:根据该同学的实验,试回答以下问题:
(1)上表第四次实验中合金里的铁恰好与稀硫酸完全反应,则其中X= Y= .
(2)该铜铁合金中铁的质量分数是多少?
(3)所加稀硫酸溶质质量分数为多少?(结果保留至0.1%).
| 第一次 | 第二次 | 第三次 | 第四次 | |
| 所取合金的质量∕g | 10 | 10 | 20 | 30 |
| 所加稀硫酸的质量∕g | 100 | 120 | 80 | X |
| 生成氢气的质量∕g | 0.2 | 0.2 | 0.2 | Y |
(2)该铜铁合金中铁的质量分数是多少?
(3)所加稀硫酸溶质质量分数为多少?(结果保留至0.1%).
某同学为探究铁合金中铁的质量分数,先后进行了三次实验,实验数据如下表:
根据该同学的实验,试回答以下问题:
(1)上表三次实验中,合金里的铁恰好完全反应时,消耗稀硫酸溶液的质量是______g.
(2)该铜铁合金中铁的质量分数是多少?______;
(3)第三次实验所得溶液溶质质量分数为______(结果保留至0.1%).
| 实验次数 项目 | 第一次 | 第二次 | 第三次 |
| 所取合金的质量/g | 20 | 20 | 40 |
| 所加稀硫酸的质量/g | 100 | 120 | 80 |
| 生成氢气的质量/g | 0.4 | 0.4 | 0.4 |
(1)上表三次实验中,合金里的铁恰好完全反应时,消耗稀硫酸溶液的质量是______g.
(2)该铜铁合金中铁的质量分数是多少?______;
(3)第三次实验所得溶液溶质质量分数为______(结果保留至0.1%).
某同学为探究铁合金中铁的质量分数,先后进行了三次实验,实验数据如下表:
根据该同学的实验,试回答以下问题:
(1)上表三次实验中,合金里的铁恰好完全反应时,消耗稀硫酸溶液的质量是______g.
(2)该铜铁合金中铁的质量分数是多少?______;
(3)第三次实验所得溶液溶质质量分数为______(结果保留至0.1%).
| 实验次数 项目 | 第一次 | 第二次 | 第三次 |
| 所取合金的质量/g | 20 | 20 | 40 |
| 所加稀硫酸的质量/g | 100 | 120 | 80 |
| 生成氢气的质量/g | 0.4 | 0.4 | 0.4 |
(1)上表三次实验中,合金里的铁恰好完全反应时,消耗稀硫酸溶液的质量是______g.
(2)该铜铁合金中铁的质量分数是多少?______;
(3)第三次实验所得溶液溶质质量分数为______(结果保留至0.1%).
某同学为探究铁合金中铁的质量分数,先后进行了三次实验,实验数据如下表:
根据该同学的实验,试回答以下问题:
(1)上表三次实验中,合金里的铁恰好完全反应时,消耗稀硫酸溶液的质量是______g.
(2)该铜铁合金中铁的质量分数是多少?______;
(3)第三次实验所得溶液溶质质量分数为______(结果保留至0.1%).
| 实验次数 项目 | 第一次 | 第二次 | 第三次 |
| 所取合金的质量/g | 20 | 20 | 40 |
| 所加稀硫酸的质量/g | 100 | 120 | 80 |
| 生成氢气的质量/g | 0.4 | 0.4 | 0.4 |
(1)上表三次实验中,合金里的铁恰好完全反应时,消耗稀硫酸溶液的质量是______g.
(2)该铜铁合金中铁的质量分数是多少?______;
(3)第三次实验所得溶液溶质质量分数为______(结果保留至0.1%).