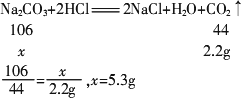��Ŀ����
һƿ���õ��������ƹ����Ѿ������˱��ʣ�ij�о���ѧϰС��Ϊ��̽�����ʳ̶ȣ��������²��룺���ܲ��ֱ��ʣ�������NaOH��Na2CO3�Ļ�������ȫ�����ʣ�������Na2CO3��
(1)���ȶԹ���ijɷֽ���ȷ����ȡ�����������Թ��У���ˮ����ܽ⣬�ȼ���������BaCl2��Һ��������ɫ���������ú�ȡ�ϲ���Һ���ټ���CuSO4��Һ��������ɫ��״����������ʵ������ȷ���ù�����________��
(2)��ȡ10.6 g�ù�����Ʒ����ƿ�У�����һ������������ϡ���ᣬֱ���������õ����������±���
�������Ʒ��Na2CO3������������
(3)��ȡ������Ʒ����ˮ������һ������������ϡ���ᣬֱ���������������ϡ��������������CO2�����������ϵ��ͼ��ʾ����ͼ�п����жϣ��ڸ���Ʒ��Һ�м���ϡ���ᣬ������֮��Ӧ��������________��

(4)���������һ��������NaOH���壬����ǰ������ͬ����������ϡ���ᷴӦ������ǰ�����������________���ʺ������������(����ڡ�����С�ڡ����ڡ�)��
��Һ�е�������ʲô
��������������ֳ����ġ���Ҫ���ᣬ��ˮ��Һ�ж��ܽ����H+���������������ƵĻ�ѧ���ʣ���������ϡ���ᡢϡ����Ļ�ѧ���ʿ�˳������й����̽���⣮
������
|
�����𰸣�(1)NaOH��Na2CO3�Ļ���� ����(2)�⣺����CO2������Ϊ148.5 g��146.3 g��2.2 g ���������Ʒ��Na2CO3������Ϊx�� ���� ������Ʒ��Na2CO3��������Ϊ�� ����(3)NaOH ����(4)���� ����������������һ������NaOH���ʵ�̽���Լ����⣮(1)BaCl2������NaOH��Ӧ��������Na2CO3��Ӧ����BaCO3��ɫ��������������ʵ�������֪��������һ������Na2CO3������������BaCl2��Һ�ܽ�Na2CO3��ȥ���ٸ����ϲ���Һ�м���CuSO4��Һ�ܲ�����ɫ��״��������֪��Һ��һ������NaOH����ˣ��ù�����NaOH��Na2CO3�Ļ���(2)ϡ������Na2CO3��Ӧ����CO2�����������غ㶨�ɣ���Ӧ����Һ�������ȷ�Ӧǰ��Һ�������٣����ٵ�����Ϊ���ɵ�CO2��������Ȼ�����û�ѧ����ʽ�������Ʒ��Na2CO3���������������Na2CO3������������(3)�۲�ͼ���֪��������ϡ������������a gʱ�Ų���CO2��˵�������ϡ��������NaOH������Ӧ������NaOH�����������Na2CO3��Ӧ����CO2��(4)ʵ������з���������Ӧ��NaOH��HCl �������������⽫���Է����붨�������ں���һ�𣬲������������û�ѧ����ʽ�ļ��㣬Ҳ����������ε����ʡ���ѧ��Ӧǰ�����ʵ��������غ��Լ�Ԫ�������غ��֪ʶ����ͬѧ�ǵ�̽����˼ά��������˽ϸߵ�Ҫ�� |