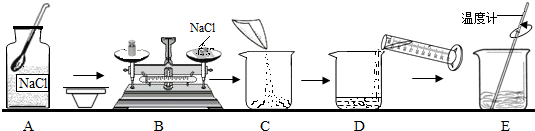
��Ŀ����
20����ͼ������50g��������Ϊ5% ���Ȼ�����Һ�IJ�������ʾ��ͼ��

�Իش�
��1��B������Ӧ�����Ȼ��Ƶ�������
��2��D����Ӧѡ��
��3��E������������

�Իش�
��1��B������Ӧ�����Ȼ��Ƶ�������
2.5
g���ź������������������ϼ��Ȼ���ʱ����ָ��ƫ��ֶ��̵���ߣ�Ӧ���еIJ�����
��������ҩƷ����ƽ��
����2��D����Ӧѡ��
100
mL ����Ͳ����10mL��100mL��ѡ����ˮʱ����Ͳ����ƽ�ţ�����Ҫ����Ͳ��Һ���
���
������ˮƽ����3��E������������
�����Ȼ����ܽ�
������������50g��������Ϊ5%���Ȼ�����Һ��������������������ʽ�ɼ���������Ȼ��Ƶ�������ˮ���������Ҫ�Ȼ��Ƶ�����Ϊ50g��5%=2.5g��ˮ�����Ϊ47.5mL�������Ȼ���ʱָ��ƫ��ֶ��̵���ߣ�˵��ҩƷ���ˣ�Ӧ�ʵ����٣�
����⣺��1��B����Ϊ��ȡ�����Ȼ��Ƶ����������ݹ�ʽ�ɼ�����Ҫ�Ȼ��Ƶ�����Ϊ50g��5%=2.5g������ʱ�Ȼ��Ʒ������̣�ָ��ƫ��ֶ��̵����˵���Ȼ��ƼӶ��ˣ�Ӧ��������ҩƷ���������Ա����Ϊ��2.5����������ҩƷ����ƽ�⣻
��2������������ˮ�����Ϊ47.5mL������Ӧ��ѡ��100mL��Ͳ����ȡ��ʹ����Ͳ����ʱ��Ҫƽ��Һ�尼Һ�����ʹ������Ա����Ϊ��100����ͣ�
��3��E����Ϊ�ܽ⣬ʹ�ò����������ÿ��Լӿ�ʳ�ε��ܽ⣬���Ա����Ϊ�������Ȼ����ܽ⣮
��2������������ˮ�����Ϊ47.5mL������Ӧ��ѡ��100mL��Ͳ����ȡ��ʹ����Ͳ����ʱ��Ҫƽ��Һ�尼Һ�����ʹ������Ա����Ϊ��100����ͣ�
��3��E����Ϊ�ܽ⣬ʹ�ò����������ÿ��Լӿ�ʳ�ε��ܽ⣬���Ա����Ϊ�������Ȼ����ܽ⣮
���������⿼������Һ�����ƣ���ɴ�����Ŀ���������ݿα�����֪ʶ���лش�����Ҫ��ͬѧ����ƽʱ��ѧϰ��Ҫ��ǿ֪ʶ�Ĵ������ܹ�����Ӧ�ã�

��ϰ��ϵ�д�
�����Ŀ