��Ŀ����
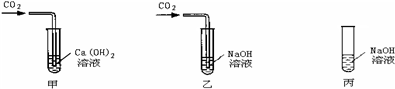
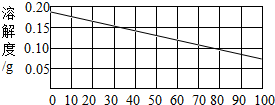
��ʯ�ҵ��ܽ������ͼ��ʾ��ijͬѧ��30��ʱ�ù�����ʯ�������˱��ͳ���ʯ��ˮ��Һ��ȡ������Һ���Թ��У��μӷ�̪�Լ�����Һ���ɫ��
��1���μӷ�̪�Լ�����Һ���ɫ��˵����Һ��________�ԣ����ᡢ��У���
��2��30��ʱ��100gˮ���ܽ���ʯ�ҵ�����Ϊ________
��3������Һ���¶ȴ�30��Ѹ������100�棬�����Һ________
A�����DZ�����Һ���������� B���Dz�������Һ����������C���������Ƶ���������
D���������Ƶ�������С���� E���������Ƶ���������
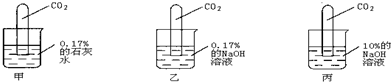
��4��ʢ�б��ͳ���ʯ��ˮ��Һ���ձ��ڿ������һ��ʱ�����ȴ�����£��ձ����Ϻ��ձ��ײ��а�ɫ���壻ȡ�������ձ��е���Һ���μӷ�̪�Լ�����Һ���ɫ�������Ʋ⣺��ɫ����ijɷֿ�����________������Һ���ɫ��ԭ����________��
�⣺��1����������������Һ��OH-����Һ�ʼ��ԣ���˿�������ɫ��̪��죬�ʴ�Ϊ������
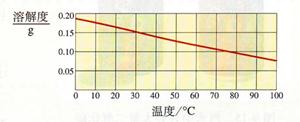
��2�������������Ƶ��ܽ������ͼ�����Ѳ������30��ʱ���������Ƶ��ܽ����0.15g������100��ˮ������ܽ�0.15g�������ƣ��ʴ�Ϊ��0.15g
��3�������������Ƶ��ܽ�����¶ȵ����߶���С����˽��������Ƶı�����Һ�ɴ�30��Ѹ������100�棬��Һ�оͻ��о��������������Һ�е����ʾͻ���٣�����Һ�����ʵ���������Ҳ���С���ʴ�Ϊ��A��D
��4�������������Ƶ��ܽ�����¶ȵ����߶���С����˸�������Һ���ʱ���ͻ����������ƴ���Һ�нᾧ���������������Ʋ������Һ��ȫ���ᾧ�������ʴ�Ϊ��Ca��OH��2����Һ������δ�ᾧ�������������ƣ��Ӷ���Һ�ʼ��ԣ�
�����������������Ƶ��ܽ�����߿�֪���ٲ������������ij�¶��µ��ܽ�ȣ����������Ƶ��ܽ�����¶ȵ����߶���С���¶����ߣ��ܽ�Ⱦͻ��С��������Һ�ͻ��о����������Ӷ�������Һ����һϵ�еı仯��������ܽ����ָ��һ�����¶��£�ij����������100���ܼ��дﵽ����״̬ʱ���ܽ��������
�����������ѶȲ��Ǻܴ���Ҫ�����˹����ܽ�����ߵ���������ܽ�ȵĸ������ѧ���������⡢��������������
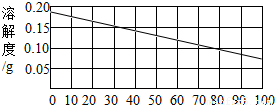
��2�������������Ƶ��ܽ������ͼ�����Ѳ������30��ʱ���������Ƶ��ܽ����0.15g������100��ˮ������ܽ�0.15g�������ƣ��ʴ�Ϊ��0.15g
��3�������������Ƶ��ܽ�����¶ȵ����߶���С����˽��������Ƶı�����Һ�ɴ�30��Ѹ������100�棬��Һ�оͻ��о��������������Һ�е����ʾͻ���٣�����Һ�����ʵ���������Ҳ���С���ʴ�Ϊ��A��D
��4�������������Ƶ��ܽ�����¶ȵ����߶���С����˸�������Һ���ʱ���ͻ����������ƴ���Һ�нᾧ���������������Ʋ������Һ��ȫ���ᾧ�������ʴ�Ϊ��Ca��OH��2����Һ������δ�ᾧ�������������ƣ��Ӷ���Һ�ʼ��ԣ�
�����������������Ƶ��ܽ�����߿�֪���ٲ������������ij�¶��µ��ܽ�ȣ����������Ƶ��ܽ�����¶ȵ����߶���С���¶����ߣ��ܽ�Ⱦͻ��С��������Һ�ͻ��о����������Ӷ�������Һ����һϵ�еı仯��������ܽ����ָ��һ�����¶��£�ij����������100���ܼ��дﵽ����״̬ʱ���ܽ��������
�����������ѶȲ��Ǻܴ���Ҫ�����˹����ܽ�����ߵ���������ܽ�ȵĸ������ѧ���������⡢��������������

��ϰ��ϵ�д�
 ������ҵ��ͬ����ϰ��ϵ�д�
������ҵ��ͬ����ϰ��ϵ�д� С��ſ�ʱ��ҵϵ�д�
С��ſ�ʱ��ҵϵ�д�
�����Ŀ
 22����ʯ�ҵ��ܽ������ͼ��ʾ��ijͬѧ��30��ʱ�ù�����ʯ�������˱��ͳ���ʯ��ˮ��Һ��ȡ������Һ���Թ��У��μӷ�̪�Լ�����Һ���ɫ��
22����ʯ�ҵ��ܽ������ͼ��ʾ��ijͬѧ��30��ʱ�ù�����ʯ�������˱��ͳ���ʯ��ˮ��Һ��ȡ������Һ���Թ��У��μӷ�̪�Լ�����Һ���ɫ��