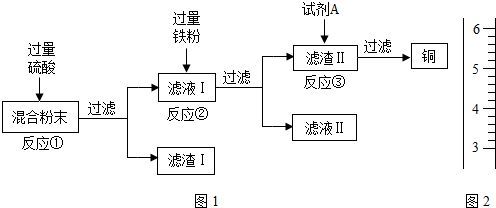
��Ŀ����
ľ̿�ۻ�ԭ����ͭʵ���Ļ�Ϸ�ĩ�к���ͭ������ͭ������ľ̿�ۣ�ʵ���ҴӸû�Ϸ�ĩ�л���ͭ�ķ�������ͼ1��
��1��д�����з�Ӧ�Ļ�ѧ����ʽ��
��Ӧ�٣�______��
��Ӧ�ڣ�______��
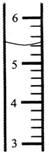
��2����Ӧ�����õ��������������Ϊ24.5%����Ҫ����40g 24.5%�����ᣬ��______g 98%�������______gˮ��ʵ�ʲ���ʱ����lOmL��Ͳȡ98%�����ᣨ�ܶ�Ϊ1.84g/cm3��������ͼ2�л�����ȡ�����Һ�森
��3���ڽ��С���Ӧ�١�������Ӧ�ڡ�������Ӧ�ۡ��IJ���ʱ���ɽ���Ӧ�����______�У����������ƣ���Ȼ��______ ����������ƣ���ʹ��Ӧ��ֽ��У�
��4���Լ�A��ѡ��______��Һ����һ�����ʵĻ�ѧʽ����
��5��Ϊ�˴ӻ�Ϸ�ĩ�л��ո����ͭ���ɶ�ʵ�鷽�������ĺ����ƣ���Ľ����ǣ����һ�����ɣ�______��

��2�������������������ļ��㹫ʽ����ʽ���η������
��3���ձ��������϶��Լ����з�Ӧ������
��4��Ҫ��ȥͭ���е����ۣ��ɼ���ϡ����
��5��Ҫ�õ������ͭ���ɽ���Ӧ�����е�ͭȫ������
���
 �⣺��1������ͭ�����ᷴӦ��������ͭ��ˮ��ͭ��ľ̿�������Ӧ����ѧ����ʽΪ��H2SO4+CuO�TCuSO4+H2O���˺����Һ�м������ۣ�����ͭ�����۷�Ӧ��������������ͭ����ѧ����ʽΪ��
�⣺��1������ͭ�����ᷴӦ��������ͭ��ˮ��ͭ��ľ̿�������Ӧ����ѧ����ʽΪ��H2SO4+CuO�TCuSO4+H2O���˺����Һ�м������ۣ�����ͭ�����۷�Ӧ��������������ͭ����ѧ����ʽΪ��Fe+CuSO4�TFeSO4+Cu
��2����Ҫ98%�����������Ϊ
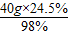 =10g������ҪŨ��������Ϊ��
=10g������ҪŨ��������Ϊ��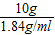 =5.4mL����Ҫˮ������Ϊ40g-10g=30g��
=5.4mL����Ҫˮ������Ϊ40g-10g=30g����3���϶����Լ��ķ�Ӧ���������ձ��У�Ϊ�˼ӿ췴Ӧ���ʿ��ò��������Ͻ��裮
��4���������к���ʣ������ۣ���˿ɼ��������ȥ
��5����ʼ��Ϸ�ĩ�е�ͭû�к��ᷴӦ�������������У�Ϊ�˻��ո����ͭ���ɽ��������е�ͭҲ����
�ʴ�Ϊ��
��1����Ӧ�٣�H2SO4+CuO�TCuSO4+H2O
��Ӧ�ڣ�Fe+CuSO4�TFeSO4+Cu
��2��10g��30g����ȡ��������Ϊ5.4mL����ͼ��ʾ��
��3���ձ����ò��������Ͻ��裮
��4��H2SO4��CuSO4�Ⱥ��������֣�
��5����������I�е�Cu
��������������ͼ����Ŀ�ؽ��ǿ���������ͼ�и����ʵ�ת����ϵ��ע��������ʵ�ȥ����γ�ȥ�������ʣ�

(13��)ľ̿�ۻ�ԭ����ͭʵ���Ļ�Ϸ�ĩ�к���ͭ������ͭ������ľ̿�ۣ�ʵ���ҴӸû�Ϸ�ĩ�л���ͭ�ķ������£�
(1)д�����з�Ӧ�Ļ�ѧ����ʽ��
��Ӧ�٣�______________________�� ��Ӧ�ڣ�___________________________��
(2)��Ӧ�����õ��������������Ϊ24.5������Ҫ����40g 24.5�������ᣬ��________g 98���������_______gˮ��
(3)�ڽ��С���Ӧ�١�������Ӧ�ڡ�������Ӧ�ۡ��IJ���ʱ���ɽ���Ӧ�����__________��(����������)��Ȼ��__________ (���������)��ʹ��Ӧ��ֽ��У�
(4)�Լ�A��ѡ��___________��Һ(��һ�����ʵĻ�ѧʽ)
(5)��֪ij�Ͻ��ĩ�����⣬����������ͭ�е�һ�ֻ����֣�ij��ȤС������ʦ��ָ���£��ԺϽ��ĩ������ͭ�Ĵ������������̽����
���������ϡ���������������Һ��Ӧ����ʽΪ2Al+2NaOH+2H2O��2NaAlO2+3H2��( ����NaAlO2����ˮ)��Fe��Cu��������������Һ��Ӧ��
���� �롿����1���úϽ��ĩ�г����⣬����������
����2���úϽ��ĩ�г����⣬������ (������)��
����3���úϽ��ĩ�г����⣬����������ͭ��
��ʵ��̽��������ʵ�����ѡ����Լ���10%���ᡢ30%NaOH��Һ��
| ʵ�鷽�� | ʵ������ | ���� |
| ��ȡһ�����ĺϽ��ĩ���ӹ�����_______����ַ�Ӧ����ˣ��������ã� | ��ĩ�����ܽ⣬��������ų��� | �Ͻ���һ������ �� |
| ��ȡ����������������ӹ�����_________����ַ�Ӧ�� | ���������ܽ⣬��������ų�����Һ��dz��ɫ�� | �Ͻ���һ������ �� |
����˼��һ����˵�����ý�������������ᷴӦ���������ᡢ��ܷ�Ӧ��˵����������������ʣ�д������ϡ���ᷴӦ�Ļ�ѧ����ʽ ��