��Ŀ����
С�ú�С������ⶨ���ᡢ������ɵĻ���������ʵ�������������Ʋ���������������̽��ʵ�飺
��ʵ��1�� ȡ20g�û���Ტ�ֳ�4�ȷݣ�Ȼ��ֱ����һ����δ֪����������BaCl2��Һ��ʵ���¼���£�
��ʵ��1�� ȡ20g�û���Ტ�ֳ�4�ȷݣ�Ȼ��ֱ����һ����δ֪����������BaCl2��Һ��ʵ���¼���£�

��ʵ��2�� ����ʵ��1���еĵ�4�ݷ�ӦҺ���ˣ�������Һ����μ���10%��NaOH��Һ��ͨ��pH�ⶨ�Ǵ�ӡ������NaOH��Һ���������ձ�����ҺpH�Ĺ�ϵ����ͼ��

�����ݴ�����С�����ݡ�ʵ��1������������������������H2SO4������������С�����ݡ�ʵ��2����������HCl�������������������£�
���⡿��������HCl����������Ϊ�ء�
HCl + NaOH �� NaCl + H2O

��ã��أ�0.1825��18.25%
���������̽�������������⡣�������õ�����Է���������H2SO4-98 BaSO4-233 BaCl2-208 ��
��1����ʵ��1���з�����Ӧ�Ļ�ѧ����ʽΪ _______________��
��2��С�ò��ԭ�������H2SO4����������Ϊ ________________��������λ��Ч���֣���
��3��С�������������HCl������������_______ ���ƫ�ߡ�����ƫ�͡�����Ӱ�족����������__________ ���硰��Ӱ�족�����ղ����𣩡�
���⡿��������HCl����������Ϊ�ء�
HCl + NaOH �� NaCl + H2O

��ã��أ�0.1825��18.25%
���������̽�������������⡣�������õ�����Է���������H2SO4-98 BaSO4-233 BaCl2-208 ��
��1����ʵ��1���з�����Ӧ�Ļ�ѧ����ʽΪ _______________��
��2��С�ò��ԭ�������H2SO4����������Ϊ ________________��������λ��Ч���֣���
��3��С�������������HCl������������_______ ���ƫ�ߡ�����ƫ�͡�����Ӱ�족����������__________ ���硰��Ӱ�족�����ղ����𣩡�
��1��BaCl2��H2SO4=2HCl��BaSO4
��2��19.6%
��3��ƫ�� �� ��ΪBaCl2��H2SO4��Ӧ�����������ɣ��Ӷ�ʹ��Һ�е��������������
��2��19.6%
��3��ƫ�� �� ��ΪBaCl2��H2SO4��Ӧ�����������ɣ��Ӷ�ʹ��Һ�е��������������

��ϰ��ϵ�д�
�����Ŀ
С�ú�С������ⶨ���ᡢ������ɵĻ���������ʵ�������������Ʋ���������������̽��ʵ�飺
��ʵ��1��ȡ20g�û���Ტ�ֳ�4�ȷݣ�Ȼ��ֱ����һ����δ֪����������BaCl2��Һ��ʵ���¼���£�
��ʵ��2������ʵ��1���еĵ�4�ݷ�ӦҺ���ˣ�������Һ����μ���10%��NaOH��Һ��ͨ��pH�ⶨ�Ǵ�ӡ������NaOH��Һ���������ձ�����ҺpH�Ĺ�ϵ��ͼ��
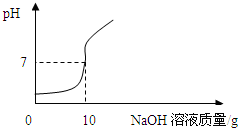
�����ݴ�����
С�����ݡ�ʵ��1����������������������
��H2SO4������������
С�����ݡ�ʵ��2����������HCl�������������������£�
�⣺��������HCl����������Ϊ�أ�
HCl+NaOH=NaCl+H2O
36.5 40
���� 10g��10%
����=0��1825=18.25%
���������̽�������������⣮�������õ�����Է���������H2SO4-98BaSO4-233 BaCl2-208 ��
��1����ʵ��1���з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2��С�ò��ԭ�������H2SO4����������Ϊ ��������λ��Ч���֣���
��3��С�������������HCl������������ ���ƫ�ߡ�����ƫ�͡�����Ӱ�족����������
���硰��Ӱ�족�����ղ����𣩣�
��ʵ��1��ȡ20g�û���Ტ�ֳ�4�ȷݣ�Ȼ��ֱ����һ����δ֪����������BaCl2��Һ��ʵ���¼���£�
| ��1�� | ��2�� | ��3�� | ��4�� | |
| ����BaCl2 ��Һ������/g |
15 | 20 | 25 | 30 |
| ��Ӧ�õ��ij���������/g | 1.398 | 1.864 | 2.330 | 2.330 |

�����ݴ�����
С�����ݡ�ʵ��1����������������������
��H2SO4������������
С�����ݡ�ʵ��2����������HCl�������������������£�
�⣺��������HCl����������Ϊ�أ�
HCl+NaOH=NaCl+H2O
36.5 40
| 20g |
| 4 |
����=0��1825=18.25%
���������̽�������������⣮�������õ�����Է���������H2SO4-98BaSO4-233 BaCl2-208 ��
��1����ʵ��1���з�����Ӧ�Ļ�ѧ����ʽΪ
��2��С�ò��ԭ�������H2SO4����������Ϊ
��3���������������HCl������������
���硰��Ӱ�족�����ղ����𣩣�
С�ú�С������ⶨ���ᡢ������ɵĻ���������ʵ�������������Ʋ���������������̽��ʵ�飺
��ʵ��1��ȡ20g�û���Ტ�ֳ�4�ȷݣ�Ȼ��ֱ����һ����δ֪����������BaCl2��Һ��ʵ���¼���£�
| ��1�� | ��2�� | ��3�� | ��4�� | |
| ����BaCl2 ��Һ������/g | 15 | 20 | 25 | 30 |
| ��Ӧ�õ��ij���������/g | 1.398 | 1.864 | 2.330 | 2.330 |

�����ݴ�����
С�����ݡ�ʵ��1����������������������
��H2SO4������������
С�����ݡ�ʵ��2����������HCl�������������������£�
�⣺��������HCl����������Ϊ�أ�
HCl+NaOH=NaCl+H2O
36.5������ 40
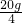 ���ء� 10g��10%
���ء� 10g��10%��ã���=0��1825=18.25%
���������̽�������������⣮�������õ�����Է���������H2SO4-98BaSO4-233�� BaCl2-208 ��
��1����ʵ��1���з�����Ӧ�Ļ�ѧ����ʽΪ______��
��2��С�ò��ԭ�������H2SO4����������Ϊ______��������λ��Ч���֣���
��3��С�������������HCl������������______���ƫ�ߡ�����ƫ�͡�����Ӱ�족����������______
���硰��Ӱ�족�����ղ����𣩣�
28����2007?��ɽ��С�ú�С������ⶨ���ᡢ������ɵĻ���������ʵ�������������Ʋ���������������̽��ʵ�飺
��ʵ��1��ȡ20g�û���Ტ�ֳ�4�ȷݣ�Ȼ��ֱ����һ����δ֪����������BaCl2��Һ��ʵ���¼���£�
��ʵ��2������ʵ��1���еĵ�4�ݷ�ӦҺ���ˣ�������Һ����μ���10%��NaOH��Һ��ͨ��pH�ⶨ�Ǵ�ӡ������NaOH��Һ���������ձ�����ҺpH�Ĺ�ϵ��ͼ��
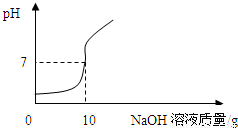
�����ݴ�����
С�����ݡ�ʵ��1����������������������
��H2SO4������������
С�����ݡ�ʵ��2����������HCl�������������������£�
�⣺��������HCl����������Ϊ�أ�
HCl+NaOH=NaCl+H2O
36.5 40
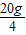 ×�� 10g×10%
×�� 10g×10%
����=0��1825=18.25%
���������̽�������������⣮�������õ�����Է���������H2SO4-98BaSO4-233 BaCl2-208 ��
��1����ʵ��1���з�����Ӧ�Ļ�ѧ����ʽΪ______��
��2��С�ò��ԭ�������H2SO4����������Ϊ______��������λ��Ч���֣���
��3��С�������������HCl������������______���ƫ�ߡ�����ƫ�͡�����Ӱ�족����������______
���硰��Ӱ�족�����ղ����𣩣�
��ʵ��1��ȡ20g�û���Ტ�ֳ�4�ȷݣ�Ȼ��ֱ����һ����δ֪����������BaCl2��Һ��ʵ���¼���£�
| ��1�� | ��2�� | ��3�� | ��4�� | |
| ����BaCl2 ��Һ������/g | 15 | 20 | 25 | 30 |
| ��Ӧ�õ��ij���������/g | 1.398 | 1.864 | 2.330 | 2.330 |

�����ݴ�����
С�����ݡ�ʵ��1����������������������
��H2SO4������������
С�����ݡ�ʵ��2����������HCl�������������������£�
�⣺��������HCl����������Ϊ�أ�
HCl+NaOH=NaCl+H2O
36.5 40
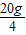 ×�� 10g×10%
×�� 10g×10%��ã���=0��1825=18.25%
���������̽�������������⣮�������õ�����Է���������H2SO4-98BaSO4-233 BaCl2-208 ��
��1����ʵ��1���з�����Ӧ�Ļ�ѧ����ʽΪ______��
��2��С�ò��ԭ�������H2SO4����������Ϊ______��������λ��Ч���֣���
��3��С�������������HCl������������______���ƫ�ߡ�����ƫ�͡�����Ӱ�족����������______
���硰��Ӱ�족�����ղ����𣩣�


