��Ŀ����
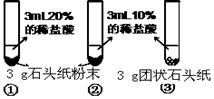
��ѧС��ͬѧ�ڼ��ȶ���������Ʒʱ�����������ݲ�������ͼһ��ʾ�����������Ƕ�����쳣���������̽����
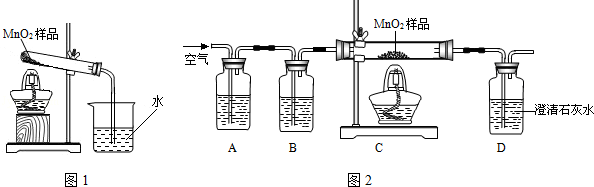
��1��������ɷֵ�̽�������ȶ���������Ʒ����ȼ�ŵ�ľ�������Թܿڣ�ľ��Ϩ�𡣽�����ͨ������ʯ��ˮ������ʯ��ˮ����ǣ������������� ��
��2����������Դ��̽����
��ͬѧ��Ϊ���������������Թ��еĿ������������ʵ��֤���ü��費����
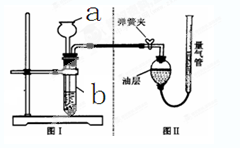
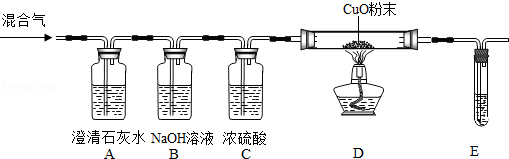
��ͬѧ��Ϊ������������Ʒ�п��ܻ���̿�ۣ�̿�۷�����Ӧ�����˸����塣���������ͼ����ʾ��ʵ������о�������Bװ�õ������Ǽ���A�з�Ӧ�Ƿ���ȫ��B�е��Լ��� ��ʵ���й۲쵽D������ʯ��ˮ����ǡ������õ�����������������������ʵ���е��κη�Ӧ�����ظ�����ʵ�飬����D�������ʯ��ˮҲ����ǡ�ͨ����ͬѧ��ʵ��֤�������������л���̿�ۣ�̿���� ��Ӧ�������������塣

��1��������ɷֵ�̽�������ȶ���������Ʒ����ȼ�ŵ�ľ�������Թܿڣ�ľ��Ϩ�𡣽�����ͨ������ʯ��ˮ������ʯ��ˮ����ǣ������������� ��
��2����������Դ��̽����
��ͬѧ��Ϊ���������������Թ��еĿ������������ʵ��֤���ü��費����
| ʵ�鲽�輰���� | ���� |
| ���ȿյ��Թܣ���һ�˵ij���ʯ��ˮû�б���� | ���� ������������������� |
��ͬѧ��Ϊ������������Ʒ�п��ܻ���̿�ۣ�̿�۷�����Ӧ�����˸����塣���������ͼ����ʾ��ʵ������о�������Bװ�õ������Ǽ���A�з�Ӧ�Ƿ���ȫ��B�е��Լ��� ��ʵ���й۲쵽D������ʯ��ˮ����ǡ������õ�����������������������ʵ���е��κη�Ӧ�����ظ�����ʵ�飬����D�������ʯ��ˮҲ����ǡ�ͨ����ͬѧ��ʵ��֤�������������л���̿�ۣ�̿���� ��Ӧ�������������塣
��1��������̼����2��������������ʯ��ˮ����������
�����������1�����ݶ�����̼��ʹ����ʯ��ˮ����ǵ����ԣ���֪�����Ƕ�����̼��
��2�����ȿյ��Թܣ���һ�˵ij���ʯ��ˮû�б���ǣ�˵�����岻�������ڿ�����ͼ����A�dz�ȥ�����еĶ�����̼������������������Һ��B����֤������̼�Ƿ��������D��ʯ��ˮ����ǣ�˵����ͬѧ�ļ���������Ӷ��õ�̼����������̷�Ӧ�����˶�����̼��

��ϰ��ϵ�д�
�����Ŀ